Comparison of Policosanols via Incorporation into Reconstituted High-Density Lipoproteins: Cuban Policosanol (Raydel®) Exerts the Highest Antioxidant, Anti-Glycation, and Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results
2.1. Ingredient Composition Analysis
2.2. Synthesis of rHDL with Policosanol
2.3. Reconstituted HDL Particle Analysis
2.4. Anti-Glycation Activity of rHDL
2.5. Anti-Oxidant Activity against LDL Oxidation
2.6. Antioxidant Activity against CML Toxicity in Embryo
2.7. Recovery from Acute Paralysis of CML Toxicity
2.8. Histology Analysis of Hepatic Tissue with H&E Staining
2.9. Extent of Fatty Liver Changes, ROS Production, and Apoptosis in Hepatic Tissue
2.10. Immunohistochemistry with IL-6 Analysis of Hepatic Tissue
2.11. Change in the Lipid Profile after an IP Injection of CML and Each rHDL
2.12. Change of Serum AST and ALT after Injection of CML and Each rHDL
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Purification of Lipoproteins
4.3. Purification of Human apoA-I
4.4. Reconstituted HDL Synthesis
4.5. Protein Quantifiaction
4.6. Electromobility Analysis in Agarose
4.7. Tryptophan Fluorescence Characterization in the rHDL
4.8. LDL Oxidation Assay
4.9. Electron Microscopic Examination
4.10. Glycation of HDL in the Presence of rHDL
4.11. Maintenance of Zebrafish
4.12. Microinjection of Zebrafish Embryos
4.13. Acute Inflammation in Adult Zebrafish
4.14. Blood Collection and Analysis
4.15. Histological Examination
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Milman, S.; Atzmon, G.; Crandall, J.; Barzilai, N. Phenotypes and genotypes of high density lipoprotein cholesterol in exceptional longevity. Curr. Vasc. Pharmacol. 2014, 12, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, L.; Zou, Y.; Tang, J.; Cai, J.; Wei, Y.; Qin, J.; Zhang, Z. Positive association of familial longevity with the moderate-high HDL-C concentration in Bama Aging Study. Aging 2018, 10, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, M.; Liu, J.; Wang, J.; Ren, G. Structure-based mechanism and inhibition of cholesteryl ester transfer protein. Curr. Atheroscler. Rep. 2023, 25, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Rader, D.J. Trials and Tribulations of CETP Inhibitors. Circ. Res. 2018, 122, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.G.; Wang, S.; Rosa, R.; Hubert, J.; Xu, S.; Chen, Y.; Bateman, T.; Blaustein, R.O. Impact of drug distribution into adipose on tissue function: The cholesteryl ester transfer protein (CETP) inhibitor anacetrapib as a test case. Pharmacol. Res. Perspect. 2019, 7, e00543. [Google Scholar] [CrossRef]
- Sitarz, R.; Juchnowicz, D.; Karakuła, K.; Forma, A.; Baj, J.; Rog, J.; Karpiński, R.; Machrowska, A.; Karakuła-Juchnowicz, H. Niacin Skin Flush Backs—From the Roots of the Test to Nowadays Hope. J. Clin. Med. 2023, 12, 1879. [Google Scholar] [CrossRef]
- Lim, S.M.; Yoo, J.A.; Lee, E.Y.; Cho, K.H. Enhancement of High-Density Lipoprotein Cholesterol Functions by Encapsulation of Policosanol Exerts Anti-Senescence and Tissue Regeneration Effects Via Improvement of Anti-Glycation, Anti-Apoptosis, and Cholesteryl Ester Transfer Inhibition. Rejuvenation Res. 2016, 19, 59–70. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Komatsu, T.; Uehara, Y. Protection of Liver Functions and Improvement of Kidney Functions by Twelve Weeks Consumption of Cuban Policosanol (Raydel®) with a Decrease of Glycated Hemoglobin and Blood Pressure from a Randomized, Placebo-Controlled, and Double-Blinded Study with Healthy and Middle-Aged Japanese Participants. Life 2023, 13, 1319. [Google Scholar] [CrossRef]
- Arruzazabala, M.; Carbajal, D.; Mas, R.; Garcia, M.; Fraga, V. Effects of Policosanol on platelet aggregation in rats. Thromb. Res. 1993, 69, 321–327. [Google Scholar] [CrossRef]
- Canavaciolo, V.L.G.; Gómez, C.V. “Copycat-policosanols” versus genuine policosanol. Rev. CENIC Cienc. Químicas 2007, 38, 207–213. [Google Scholar]
- Lee, H.G.; Woo, S.Y.; Ahn, H.J.; Yang, J.Y.; Lee, M.J.; Kim, H.Y.; Song, S.Y.; Lee, J.H.; Seo, W.D. Comparative Analysis of Policosanols Related to Growth Times from the Seedlings of Various Korean Oat (Avena sativa L.) Cultivars and Screening for Adenosine 5′-Monophosphate-Activated Protein Kinase (AMPK) Activation. Plants 2022, 11, 1844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, C.; Sun, L.; He, Z.; Feng, Y.; Li, X.; Gan, J.; Chen, X. Effect of policosanol from insect wax on amyloid β-peptide-induced toxicity in a transgenic Caenorhabditis elegans model of Alzheimer’s disease. BMC Complement. Med. Ther. 2021, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, A.; Brighenti, V.; Mascolo, D.; Pellati, F.; Costi, M.P. A new strategy based on microwave-assisted technology for the extraction and purification of beeswax policosanols for pharmaceutical purposes and beyond. J. Pharm. Biomed. Anal. 2019, 172, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Kaup, R.M.; Khayyal, M.T.; Verspohl, E.J. Antidiabetic Effects of a Standardized Egyptian Rice Bran Extract. Phytother. Res. 2013, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-T.; Ismail, M.; Tohit, E.R.M.; Abdullah, R.; Zhang, Y.-D. Attenuation of Thrombosis by Crude Rice (Oryza sativa) Bran Policosanol Extract: Ex Vivo Platelet Aggregation and Serum Levels of Arachidonic Acid Metabolites. Evid. -Based Complement. Altern. Med. 2016, 2016, 7343942. [Google Scholar] [CrossRef]
- Cho, K.-H.; Baek, S.H.; Nam, H.-S.; Kim, J.-E.; Kang, D.-J.; Na, H.; Zee, S. Cuban Sugar Cane Wax Alcohol Exhibited Enhanced Antioxidant, Anti-Glycation and Anti-Inflammatory Activity in Reconstituted High-Density Lipoprotein (rHDL) with Improved Structural and Functional Correlations: Comparison of Various Policosanols. Int. J. Mol. Sci. 2023, 24, 3186. [Google Scholar] [CrossRef]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. Int. Rev. J. 2017, 8, 54–62. [Google Scholar] [CrossRef]
- Suárez, G.; Rajaram, R.; Oronsky, A.L.; Gawinowicz, M.A. Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose. J. Biol. Chem. 1989, 264, 3674–3679. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased toll-like receptor (tlr) activation and tlr ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef]
- Wattrus, S.J.; Zon, L.I. Blood in the water: Recent uses of zebrafish to study myeloid biology. Curr. Opin. Hematol. 2021, 28, 43–49. [Google Scholar] [CrossRef]
- Abbate, F.; Maugeri, A.; Laurà, R.; Levanti, M.; Navarra, M.; Cirmi, S.; Germanà, A. Zebrafish as a Useful Model to Study Oxidative Stress-Linked Disorders: Focus on Flavonoids. Antioxidants 2021, 10, 668. [Google Scholar] [CrossRef]
- Cho, K.H.; Nam, H.S.; Kim, J.E.; Na, H.J.; del Carmen Dominguez-Horta, M.; Martinez-Donato, G. CIGB-258 Exerts Potent Anti-Inflammatory Activity against Carboxymethyllysine-Induced Acute Inflammation in Hyperlipidemic Zebrafish via the Protection of Apolipoprotein A-I. Int. J. Mol. Sci. 2023, 24, 7044. [Google Scholar] [CrossRef]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dhindsa, D.; Almuwaqqat, Z.; Sun, Y.V.; Quyyumi, A.A. Very High High-Density Lipoprotein Cholesterol Levels and Cardiovascular Mortality. Am. J. Cardiol. 2022, 167, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, S.-J.; Yadav, D.; Kim, J.-R. Consumption of Cuban Policosanol Improves Blood Pressure and Lipid Profile via Enhancement of HDL Functionality in Healthy Women Subjects: Randomized, Double-Blinded, and Placebo-Controlled Study. Oxidative Med. Cell. Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-Y.; Yoo, J.-A.; Lim, S.-M.; Cho, K.-H. Anti-Aging and Tissue Regeneration Ability of Policosanol Along with Lipid-Lowering Effect in Hyperlipidemic Zebrafish via Enhancement of High-Density Lipoprotein Functionality. Rejuvenation Res. 2016, 19, 149–158. [Google Scholar] [CrossRef]
- Qiao, Q.; Bouwman, F.G.; van Baak, M.A.; Roumans, N.J.T.; Vink, R.G.; Mariman, E.C.M. Plasma Levels of Triglycerides and IL-6 Are Associated With Weight Regain and Fat Mass Expansion. J. Clin. Endocrinol. Metab. 2022, 107, 1920–1929. [Google Scholar] [CrossRef]
- Toader, M.P.; Taranu, T.; Constantin, M.M.; Olinici, D.; Mocanu, M.; Costan, V.V.; Toader, S. High serum level of interleukin-6 is linked with dyslipidemia in oral lichen planus. Exp. Ther. Med. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Pietrzak, A.; Chabros, P.; Grywalska, E.; Pietrzak, D.; Kandzierski, G.; Wawrzycki, B.; Roliński, J.; Gawęda, K.; Krasowska, D. Serum concentration of interleukin 6 is related to inflammation and dyslipidemia in patients with psoriasis. Adv. Dermatol. Allergol. 2020, 37, 41–45. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The Distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Brewer, H.B., Jr.; Ronan, R.; Meng, M.; Bishop, C. Isolation and characterization of apolipoproteins A-I, A-II, and A-IV. Methods Enzymol. 1986, 128, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.; Tolbert, N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [CrossRef]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Nusslein-Volhard, C.; Dahm, R. Zebrafish: A Practical Approach, 1st ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- National Research Council of the National Academy of Sciences. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 2010.
- Burris, B.; Jensen, N.; Mokalled, M.H. Assessment of Swim Endurance and Swim Behavior in Adult Zebrafish. J. Vis. Exp. 2021, 177, e63240. [Google Scholar] [CrossRef]
- Owusu-Ansah, E.; Yavari, A.; Mandal, S.; Banerjee, U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008, 40, 356–361. [Google Scholar] [CrossRef]
- Hayashi, M.; Sofuni, T.; Ishidate, M., Jr. An application of Acridine Orange fluorescent staining to the micronucleus test. Mutat. Res. Lett. 1983, 120, 241–247. [Google Scholar] [CrossRef]













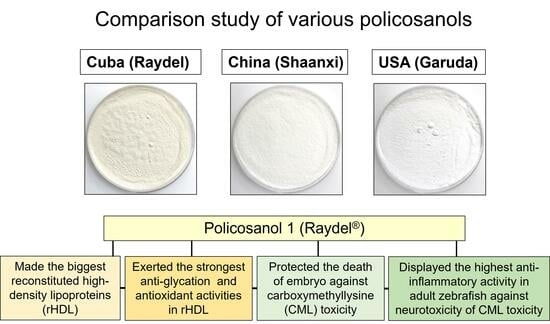
| Product Name/Description | Policosanol 1 (Raydel®) | Policosanol 2 (Shaanxi) | Policosanol 3 (Lesstanol®) | |
|---|---|---|---|---|
| Country | Cuba | China | USA | |
| Manufacturer | CNIC 1 | Shaanxi 2 | Garuda 3 | |
| Powder image |  |  |  | |
| Source | Sugar Cane Wax | Rice Bran | Sugar Cane Wax | |
| Ingredients of Long-Chain Aliphatic Alcohols | Desirable Range 4 (mg/g) | Determined Amount (mg/g) (%) 5 | Determined Amount (mg/g) (%) | Determined Amount (mg/g) (%) |
| Average molecular weight | 418 | 442 | 419 | |
| Total amount on the label | >900 | 982 | 400 | 900 |
| 1-tetracosanol (C24) | 0.1–20 | 0.3 (0.0) | 70 (9.5) | 28 (3.1) |
| 1-hexacosanol (C26) | 30–100 | 38 (3.9) | 58 (7.9) | 69 (7.7) |
| 1-heptacosanol (C27) | 1–30 | 9 (0.9) | 1 (0.1) | 8 (0.9) |
| 1-octacosanol (C28) | 600–700 | 692 (70.5) | 56 (7.6) | 546 (60.7) |
| 1-nonacosanol (C29) | 1–20 | 6 (0.6) | 6 (0.8) | 11 (1.2) |
| 1-triacotanol (C30) | 100–150 | 139 (14.2) | 213 (28.8) | 131 (14.6) |
| 1-dotriacotanol (C32) | 50–100 | 78 (7.9) | 181 (24.5) | 62 (6.9) |
| 1-tetratriacotanol (C34) | 1–50 | 20 (2.0) | 154 (20.8) | 45 (5.0) |
| Determined final total amount (mg) | more than 900 | 982 (100) | 739 (100) | 900 (100) |
| Name | Description | MW of PCO (Averaged) | Molar Ratio POPC:FC:apoA-I:PCO | WMF (nm) | Diameter (nm) |
|---|---|---|---|---|---|
| rHDL-0 | rHDL alone | - | 95:5:1:0 | 329.2 ± 1.7 | 60.7 ± 1.5 |
| rHDL-1 | Policosanol 1-rHDL | 418.0 | 95:5:1:1 | 325.2 ± 1.8 *** | 83.1 ± 3.1 *** |
| rHDL-2 | Policosanol 2-rHDL | 442.4 | 95:5:1:1 | 326.5 ± 1.5 * | 62.7 ± 2.6 * |
| rHDL-3 | Policosanol 3-rHDL | 418.8 | 95:5:1:1 | 327.3 ± 1.4 * | 60.2 ± 1.6 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Kang, D.-J.; Baek, S.-H. Comparison of Policosanols via Incorporation into Reconstituted High-Density Lipoproteins: Cuban Policosanol (Raydel®) Exerts the Highest Antioxidant, Anti-Glycation, and Anti-Inflammatory Activity. Molecules 2023, 28, 6715. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28186715
Cho K-H, Kim J-E, Nam H-S, Kang D-J, Baek S-H. Comparison of Policosanols via Incorporation into Reconstituted High-Density Lipoproteins: Cuban Policosanol (Raydel®) Exerts the Highest Antioxidant, Anti-Glycation, and Anti-Inflammatory Activity. Molecules. 2023; 28(18):6715. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28186715
Chicago/Turabian StyleCho, Kyung-Hyun, Ji-Eun Kim, Hyo-Seon Nam, Dae-Jin Kang, and Seung-Hee Baek. 2023. "Comparison of Policosanols via Incorporation into Reconstituted High-Density Lipoproteins: Cuban Policosanol (Raydel®) Exerts the Highest Antioxidant, Anti-Glycation, and Anti-Inflammatory Activity" Molecules 28, no. 18: 6715. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28186715