Determination of Tiamulin Concentration in Sow Milk and in Sera of Suckling Piglets
Abstract
:1. Introduction
2. Results
2.1. Validation of the UHPLC-MS/MS Method
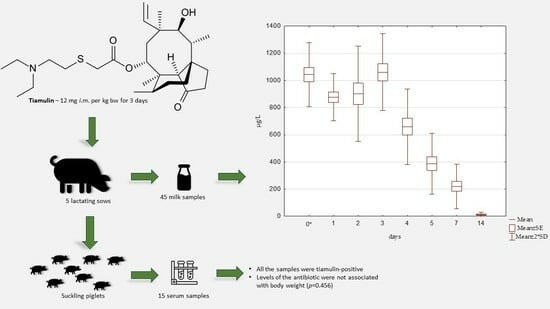
2.2. Detection and Quantification of Tiamulin
3. Discussion
4. Materials and Methods
4.1. Animal Experiment and Sample Collection
4.2. Animal Experiment and Sample Collection
4.2.1. Reagents, Chemicals, and Standards
4.2.2. Sample Preparation and UHPLC-MS/MS Analysis
4.2.3. Analytical Method Validation
4.3. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kavanagh, F.; Hervey, A.; Robbins, W.J. Antibiotic substances from Basidiomycetes: VIII. Pleurotus Multilus (Fr.) Sacc. and Pleurotus Passeckerianus Pilat. Proc. Natl. Acad. Sci. USA 1951, 37, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Knauseder, F.; Brandl, E. Pleuromutilins fermentation, structure and biosynthesis. J. Antibiot. 1976, 29, 125–131. [Google Scholar] [CrossRef]
- Laber, G.; Schütze, E. Blood level studies in chickens, turkey poults and swine with tiamulin, a new antibiotic. J. Antibiot. 1977, 30, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.J.; Holzapfel, C.W.; Rickards, R.W. The structure and some aspects of the biosynthesis of pleuromutilin. Tetrahedron 1966, 22, 359–387. [Google Scholar] [CrossRef]
- Espacenet Patent Search. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?CC=gb&NR=2027590&KC=&FT=E&locale=en_EP# (accessed on 20 February 2023).
- European Medicines Agency. Information on Medicines for Use in Animals That Are Authorised Anywhere in the European Union (EU) and European Economic Area (EEA). Available online: https://medicines.health.europa.eu/veterinary/en (accessed on 20 February 2023).
- Hodgin, L.A.; Högenauer, G. The mode of action of pleuromutilin derivatives. Effect on cell-free polypeptide synthesis. Eur. J. Biochem. 1974, 47, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, S.M.; Karlsson, M.; Johansson, L.B.; Vester, B. The pleuromutilin drugs tiamulin and valnemulin bind to the RNA at the peptidyl transferase centre on the ribosome. Mol. Microbiol. 2001, 41, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Drews, J.; Georgopoulos, A.; Laber, G.; Schütze, E.; Unger, J. Antimicrobial activities of 81.723 hfu, a new pleuromutilin derivative. Antimicrob. Agents Chemother. 1975, 7, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Chengappa, M.M.; Pace, L.W.; Williams, J.A.; Herren, C.H.; Ascher, S.E. Efficacy of tiamulin against experimentally induced Streptococcus suis type-2 infection in swine. J. Am. Vet. Med. Assoc. 1990, 197, 1467–1470. [Google Scholar]
- Baughn, C.O.; Alpaugh, W.C.; Linkenheimer, W.H.; Maplesden, D.C. Effect of tiamulin in chickens and turkeys infected experimentally with avian Mycoplasma. Avian Dis. 1978, 22, 620–626. [Google Scholar] [CrossRef]
- Burch, D.G.; Goodwin, R.F. Use of tiamulin in a herd of pigs seriously affected with Mycoplasma hyosynoviae arthritis. Vet. Rec. 1984, 115, 594–595. [Google Scholar] [CrossRef]
- Roberts, E.; Hammer, J.M.; Lechtenberg, K.; Roycroft, L.; King, S. Investigation of tiamulin hydrogen fumerate in-feed antibiotic for the control of porcine respiratory disease complex that includes Mycoplasma hyopneumoniae. J. Swine Health Prod. 2011, 19, 218–225. [Google Scholar]
- Jordan, F.T.; Forrester, C.A.; Ripley, P.H.; Burch, D.G. In vitro and in vivo comparisons of valnemulin, tiamulin, tylosin, enrofloxacin, and lincomycin/spectinomycin against Mycoplasma gallisepticum. Avian Dis. 1998, 42, 738–745. [Google Scholar] [CrossRef]
- Elazab, S.T.; Elshater, N.S.; Hashem, Y.H.; Park, S.C.; Hsu, W.H. Tissue residues and pharmacokinetic/pharmacodynamic modelling of tiamulin against Mycoplasma anatis in ducks. Front. Vet. Sci. 2020, 27, 603950. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.; Saranpää, T.; Rautiainen, E. In vitro sensitivity of the swine Brachyspira species to tiamulin in Finland 1995–1997. Acta Vet. Scand. 1999, 40, 355–358. [Google Scholar] [CrossRef]
- Karlsson, M.; Gunnarsson, A.; Franklin, A. Susceptibility to pleuromutilins in Brachyspira (Serpulina) hyodysenteriae. Anim. Health Res. Rev. 2001, 2, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lobova, D.; Smola, J.; Cizek, A. Decreased susceptibility to tiamulin and valnemulin among Czech isolates of Brachyspira hyodysenteriae. J. Med. Microbiol. 2004, 53, 287–291. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Jensen, N.E. Susceptibility testing of Actinobacillus pleuropneumoniae in Denmark. Evaluation of three different media of MIC-determinations and tablet diffusion tests. Vet. Microbiol. 1999, 64, 299–305. [Google Scholar] [CrossRef] [PubMed]
- McOrist, S.; Gebhart, C.J. In vitro testing of antimicrobial agents for proliferative enteropathy (ileitis). Swine Health Prod. 1995, 3, 146–149. [Google Scholar]
- McOrist, S.; Mackie, R.A.; Lawson, G.H.K. Antimicrobial susceptibility of Ileal Symbiont intracellularis isolated from pigs with proliferative enteropathy. J. Clin. Microbiol. 1995, 22, 1314–1317. [Google Scholar] [CrossRef]
- Islam, K.M.; Klein, U.; Burch, D.G. The activity and compatibility of the antibiotic tiamulin with other drugs in poultry medicine—A review. Poult. Sci. 2009, 88, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.F.; Emborg, H.D.; Aarestrup, F.M. Indications and patterns of therapeutic use of antimicrobial agents in the Danish pig production from 2002 to 2008. J. Vet. Pharmacol. Ther. 2012, 35, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Van Duijkeren, E.; Greko, C.; Pringle, M.; Baptiste, K.E.; Catry, B.; Jukes, H.; Moreno, M.A.; Pomba, M.C.; Pyörälä, S.; Rantala, M.; et al. Pleuromutilins: Use in food-producing animals in the European Union, development of resistance and impact on human and animal health. J. Antimicrob. Chemother. 2014, 69, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020. Trends from 2010 to 2020. Eleventh ESVAC Report. Available online: https://www.ema.europa.eu/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2019-2020-trends-2010-2020-eleventh_en.pdf (accessed on 20 February 2023).
- Lykkeberg, A.K.; Cornett, C.; Halling-Sørensen, B.; Hansen, S.H. Isolation and structural elucidation of tiamulin metabolites formed in liver microsomes of pigs. J. Pharm. Biomed. Anal. 2006, 42, 223–231. [Google Scholar] [CrossRef]
- European Medicines Agency. EMEA/MRL/578/99-FINAL-corr.1. August 1999. Committee for Veterinary Medicinal Products. Tiamulin. Summary Report (1). Available online: https://www.ema.europa.eu/en/documents/mrl-report/tiamulin-summary-report-1-committee-veterinary-medicinal-products_en.pdf (accessed on 20 February 2023).
- Chen, H.-C.; Cheng, S.-H.; Tsai, Y.-H.; Hwang, D.-F. Determination of tiamulin residue in pork and chicken by solid phase extraction and HPLC. JFDA 2006, 14, 80–83. [Google Scholar] [CrossRef]
- Ziv, G.; Levisohn, S.L.; Bar-Moshe, B.; Bor, A.; Soback, S. Clinical pharmacology of tiamulin in ruminants. J. Vet. Pharmacol. Ther. 1983, 6, 23–32. [Google Scholar] [CrossRef]
- Nozal, M.J.; Bernal, J.L.; Martín, M.T.; Jiménez, J.J.; Bernal, J.; Higes, M. Trace analysis of tiamulin in honey by liquid chromatography-diode array-electrospray ionization mass spectrometry detection. J. Chromatogr. A 2006, 1116, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.; Cowen, T. Determination of tiamulin hydrogen fumarate in animal feeds using high-performance liquid chromatography. Analyst 1982, 107, 319–323. [Google Scholar] [CrossRef]
- Moore, D.B.; Britton, N.L.; Smallidge, R.L.; Riter, K.L. Determination of tiamulin in type C medicated swine feeds using high throughput extraction with liquid chromatography. J. AOAC Int. 2002, 85, 533–540. [Google Scholar] [CrossRef]
- Krasucka, D.; Mitura, A.; Cybulski, W.; Kos, K.; Pietroń, W. Tiamulin hydrogen fumarate-veterinary uses and HPLC method of determination in premixes and medicated feeding stuffs. Acta Pol. Pharm. 2010, 67, 682–685. [Google Scholar]
- Schlüsener, M.P.; Bester, K. Persistence of antibiotics such as macrolides, tiamulin and salinomycin in soil. Environ. Pollut. 2006, 143, 565–571. [Google Scholar] [CrossRef]
- Schlüsener, M.P.; Bester, K.; Spiteller, M. Determination of antibiotics such as macrolides, ionophores and tiamulin in liquid manure by HPLC-MS/MS. Anal. Bioanal. Chem. 2003, 375, 942–947. [Google Scholar] [CrossRef]
- Ben, W.; Qiang, Z.; Adams, C.; Zhang, H.; Chen, L. Simultaneous determination of sulfonamides, tetracyclines and tiamulin in swine wastewater by solid-phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A 2008, 1202, 173–180. [Google Scholar] [CrossRef]
- Dimitrova, D.; Katsarov, V.; Dimitrov, D.; Tsoneva, D. Pharmacokinetics of tiamulin and chlortetracycline after application of tetramutin-premix in pigs. Agric. Sci. Technol. 2011, 3, 229–234. [Google Scholar]
- Product Description. Available online: https://www.vetoquinol.pl/products/tiamowet-injr (accessed on 20 February 2023).
- Po, H.N.; Senozan, N.M. The Henderson-Hasselbalch Equation: Its History and Limitations. J. Chem. Educ. 2001, 78, 1499–1503. [Google Scholar] [CrossRef]
- Larsen, L. Effect of subinhibitory concentrations of tiamulin on the haemagglutinating properties of fimbriated Escherichia coli (K88, K99). Res. Vet. Sci. 1988, 45, 134–135. [Google Scholar] [CrossRef]
- Tran, H.; Nguyen, L.; Ogle, B.; Lindberg, J. Survey on the prevalence of diarrhoea in pre-weaning piglets and on feeding systems as contributing risk factors in smallholdings in Central Vietnam. Trop. Anim. Health Prod. 2006, 38, 397–405. [Google Scholar]
- Mesonero-Escuredo, S.; Strutzberg-Minder, K.; Casanovas, C.; Segales, J. Viral and bacterial investigations on the aetiology of recurrent pig neonatal diarrhoea cases in Spain. Porc. Health Manag. 2018, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Eleazar, A.N.; Sonibare, A.O.; Ojo, O.E.; Awoyomi, O.J.; Otesile, E.B. A Survey of Neonatal Piglet Mortality in Commercial Pig Farms in Lagos State, Southwest Nigeria. NVJ 2021, 42, 238–251. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to Be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC. Off. J. Eur. Union L 2021, 180, 84–109. [Google Scholar]


| Analyte | Fortification Level [µg/L] | Repeatability [%] (n = 6) | Reproducibility [%] (n = 18) | Recovery [%] (n = 18) |
|---|---|---|---|---|
| Tiamulin | Milk | |||
| 1.0 | 8.8 | 12.8 | 94.0 | |
| 5.0 | 5.6 | 5.1 | 107 | |
| 10 100 | 9.6 8.4 | 11.7 12.4 | 103 105 | |
| Serum | ||||
| 1.0 | 7.9 | 13.4 | 97.3 | |
| 5.0 | 6.6 | 8.1 | 104 | |
| 10 100 | 9.8 8.0 | 12.2 11.5 | 106 102 | |
| Species | Animals Tested | Tiamulin Dosage | Mean Peak Serum Cmax | Mean Peak Blood Cmax | Milk Cmax to Blood Cmax | Ref. |
|---|---|---|---|---|---|---|
| n | mg/kg | μg/L | μg/L | ratio | ||
| Goats | 23 | 10 | 560 | 2500 | 4.46 | [29] |
| Ewes | 8 | 10 | 640 | 7500 | 11.72 | [29] |
| Sows | 5 | 12 | 610 | 1043 | 1.72 | [this study] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cybulski, P.; Gajda, A.; Bilecka, M.; Jabłoński, A. Determination of Tiamulin Concentration in Sow Milk and in Sera of Suckling Piglets. Molecules 2023, 28, 6940. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28196940
Cybulski P, Gajda A, Bilecka M, Jabłoński A. Determination of Tiamulin Concentration in Sow Milk and in Sera of Suckling Piglets. Molecules. 2023; 28(19):6940. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28196940
Chicago/Turabian StyleCybulski, Piotr, Anna Gajda, Magdalena Bilecka, and Artur Jabłoński. 2023. "Determination of Tiamulin Concentration in Sow Milk and in Sera of Suckling Piglets" Molecules 28, no. 19: 6940. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28196940