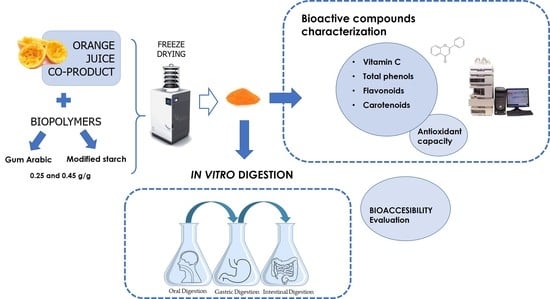
In Vitro Bioaccessibility of Bioactive Compounds of Freeze-Dried Orange Juice Co-Product Formulated with Gum Arabic and Modified Starch
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of the Presence of Biopolymers on the Bioactive Compounds Content and Antioxidant Capacity of Freeze-Dried Orange Juice Co-Product Samples
2.2. In Vitro Bioaccessibility of Bioactive Compounds from Freeze-Dried Orange Juice Co-Product Samples with and without Biopolymers
3. Materials and Methods
3.1. Materials
3.2. Preparation of Powdered Co-Product Samples
3.3. In Vitro Gastro-Intestinal Digestion
3.4. Analytical Determinations
3.4.1. Water Content
3.4.2. Total Phenolic Compounds
3.4.3. Major Flavonoids: Hesperidin and Narirutin
3.4.4. Vitamin C
3.4.5. Total Carotenoids
3.4.6. Antioxidant Activity
3.4.7. In Vitro Bioaccessibility
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Department of Agriculture Foreign Agricultural Service (USDA). Citrus: World Markets and Trade. 2022. Available online: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 15 September 2022).
- Jiménez-Castro, M.P.; Buller, L.S.; Sganzerla, W.G.; Forster-Carneiro, T. Bioenergy production from orange industrial waste: A case study. Biofuels Bioprod. Bioref. 2020, 14, 1239–1253. [Google Scholar] [CrossRef]
- European Parliament. Circular Economy: Definition, Importance and Benefits. 2021. Available online: https://www.europarl.europa.eu/doceo/document/TA-9-2021-0040_ES.pdf (accessed on 15 September 2022).
- Hussain, S.; Joudu, I.; Bhat, R. Dietary fibre from underutilised plant resources—A positive approach for the valorisation of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Domínguez, M.T. Flavonoides extraídos de la cascara de naranja tangelo (Citrus reticulata x Citrus paradisi) y su aplicación como antioxidante natural en el aceite vegetal sacha inchi (Plukenetia volubilis). Sci. Agropecu. 2016, 7, 419–431. [Google Scholar] [CrossRef]
- Escobedo-Avellaneda, Z.; Gutiérrez-Uribe, J.; Valdez-Fragoso, A.; Torres, J.A.; Welti-chanes, J. Phytochemicals and antioxidant activity of juice, flavedo, albedo and comminuted Orange. J. Funct. Foods 2014, 6, 470–481. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Dellacassa, E.; Nardin, T.; Larcher, R.; Gámbaro, A.; Medrano-Fernandez, A.; del Castillo, M.D. In Vitro Bioaccessibility of Bioactive Compounds from Citrus Pomaces and Orange Pomace Biscuits. Molecules 2021, 26, 3480. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.; Ucella, I.; Gombossy, B.D.; Isay, S.M.; de Moreno, A.; Guy, J. Bioactive compounds of fruit by-products as potential prebiotics. In Valorisation of Agri-Food Wastes and By-Products, 1st ed.; Bhat, R., Ed.; Academic Press: London, UK, 2021; pp. 47–59. [Google Scholar]
- Mohd Basri, M.S.; Abdul Karim Shah, N.N.; Sulaiman, A.; Mohamed Amin Tawakkal, I.S.; Mohd Nor, M.Z.; Ariffin, S.H.; Abdul Ghani, N.H.; Mohd Salleh, F.S. Progress in the Valorization of Fruit and Vegetable Wastes: Active Packaging, Biocomposites, By-Products, and Innovative Technologies Used for Bioactive Compound Extraction. Polymers 2021, 13, 3503. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.; García-Martínez, E.; Martínez-Navarrete, N. Protective capacity of gum Arabic, maltodextrin, different starches, and fibres on the bioactive compounds and antioxidant activity of an Orange puree (Citrus sinensis (L.) Osbeck) against freeze-drying and in vitro digestion. Food Chem. 2021, 357, 129724. [Google Scholar] [CrossRef]
- Ratti, C. Freeze drying for food powder production. In Handbook of Food Powders: Processes and Properties; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing Limited: Sawston, UK, 2013; pp. 57–84. [Google Scholar] [CrossRef]
- Telis, V.R.N.; Martínez-Navarrete, N. Biopolymers used as drying aids in spray-drying and freeze-drying of fruit juices and pulps. In Biopolymer Engineering in Food Processing, 1st ed.; Telis, V.R.N., Ed.; CRC Press: Boca Ratón, FL, USA, 2012; pp. 279–325. [Google Scholar]
- Williams, P.A.; Phillips, G.O. Gum arabic. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Oxford, UK, 2021; pp. 627–652. [Google Scholar]
- Carlsen, L.; Bruggemann, R. The 17 United Nations’ sustainable development goals: A status by 2020. Int. J. Sustain. Dev. World Ecol. 2022, 29, 3. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Agricultural Research Service (USDA). USDA National Nutrient Database for Standard Reference. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169103/nutrients (accessed on 27 October 2022).
- Camacho, M.M.; Zago, M.; García-Martínez, E.; Martínez-Navarrete, N. Free and bound phenolic compounds from powdered orange juice by-product and their contribution to antioxidant activity. Antioxidants 2022, 11, 1748. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Cánovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005; Volume 86, pp. 71–75. [Google Scholar]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, V.M.; Macedo, G.A.; Macedo, J.A. Citrus bioactive phenolics: Role in the obesity treatment. LWT Food Sci. Technol. 2014, 59, 1205–1212. [Google Scholar] [CrossRef] [Green Version]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.S.; Haruenkit, R.; Lojek, A.; Cíź, M.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Michalczyk, M.; MacUra, R.; Matuszak, I. The effect of air-drying, freeze-drying and storage on the quality and antioxidant activity of some selected berries. J. Food Process. Preserv. 2009, 33, 11–21. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo, R.G.; Chiș, M.S.; Martínez-Navarrete, N.; Camacho, M.M. Dried orange juice waste as a source of bioactive compounds. Br. Food J. 2022, 124, 4653–4665. [Google Scholar] [CrossRef]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 433, 1086–1107. [Google Scholar] [CrossRef] [Green Version]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Effect of Thermal treatment and storage on the stability of organic acids and the functional value of grapefruit Juice. Food Chem. 2010, 118, 291–299. [Google Scholar] [CrossRef]
- Hayashi, T.; Terao, A.; Ueda, S.; Namiki, M. Red pigment formation by the reaction of oxidised ascorbic acid and protein in a food model system of low moisture content. J. Biol. Chem. 1985, 49, 3139–3144. [Google Scholar] [CrossRef] [Green Version]
- Gardner, P.T.; White, T.A.C.; McPhail, D.B.; Duthie, G.G. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000, 68, 471–474. [Google Scholar] [CrossRef]
- Faulks, M.; Southon, S. Carotenoids, metabolism and disease. In Handbook of Nutraceuticals and Functional Foods; Wildman, R., Ed.; CRC Press, Inc.: Boca Ratón, FL, USA, 2001; pp. 143–156. [Google Scholar]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; Camacho, M.M.; Martínez-Navarrete, N. The impact of freeze-drying conditions on the physicochemical properties and bioactive compounds of a freeze-dried orange puree. Foods 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratianni, A.; Cinquanta, L.; Panfili, G. Degradation of carotenoids in orange juice during microwave heating. LWT—Food Sci. Technol. 2010, 43, 867–871. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Changes in vitamin C, phenolic, and carotenoid profiles throughout in vitro gastro-intestinal digestion of a blended fruit juice. J. Agric. Food Chem. 2013, 61, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Scott, G. Antioxidants in Science, Technology, Medicine, and Nutrition, 1st ed.; Woodhead Publishing: Birmingham, UK, 1997. [Google Scholar]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Stability of micronutrients and phytochemicals of grapefruit jam as affected by the obtention process. Food Sci. Technol. Int. 2016, 22, 203–212. [Google Scholar] [CrossRef]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Plaza, L.; de Ancos, B.; Cano, M.P. Quantitative bioactive compounds assessment and their relative contribution to the antioxidant capacity of commercial orange juices. J. Sci. Food Agric. 2003, 83, 430–439. [Google Scholar] [CrossRef]
- Sádecká, J.; Polovka, M.; Kolek, E.; Belajová, E.; Tobolková, B.; Daško, L.; Durec, J. Orange juice with pulp: Impact of pasteurisation and storage on flavour, polyphenols, ascorbic acid and antioxidant activity. J. Food Nutr. Res. 2014, 53, 371–388. [Google Scholar]
- Liu, D.; Shi, J.; Ibarra, A.C.; Kakuda, Y.; Xue, S.J. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT Food Sci. Technol. 2008, 41, 1344–1349. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Capanoglu, E. Evaluating the in vitro bioaccessibility of phenolics and antioxidant activity during consumption of dried fruits with nuts. LWT Food Sci. Technol. 2014, 56, 284–289. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary Fiber as a Carrier of Dietary Antioxidants: An Essential Physiological Function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, W.; Huang, H.; Ye, X.; Sun, P. Flavonoids, phenolic acids, carotenoids and antioxidant activity of fresh eating citrus.fruits, using the coupled in vitro digestion and human intestinal HepG2 cells model. Food Chem. 2019, 279, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.; Viuda-Martos, M. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastro-intestinal digestion. Ind. Crops Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Hu, Y.; Kou, G.; Chen, Q.; Li, Y.; Zhou, Z. Protection and delivery of mandarin (Citrus reticulata Blanco) peel extracts by encapsulation of whey protein concentrate nanoparticles. LWT-Food Sci. Technol. 2019, 99, 24–33. [Google Scholar] [CrossRef]
- Martínez-Navarrete, N.; García-Martínez, E.; Camacho, M.M. Characterization of the Orange Juice Powder Co-Product for Its Valorization as a Food Ingredient. Foods 2023, 12, 97. [Google Scholar] [CrossRef]
- Miller, D.D.; Schricker, B.R.; Rasmussen, R.R.; Van Campen, D. An in vitro method for estimation of iron availability from meals. Am. J. Clin. Nutr. 1981, 34, 2248–2256. [Google Scholar] [CrossRef]
- Huang, H.; Sun, Y.; Lou, S.; Li, H.; Ye, X. In vitro digestion combined with cellular assay to determine the antioxidant activity in Chinese bayberry (Myrica rubra Sieb. et Zucc.) fruits: A comparison with traditional methods. Food Chem. 2014, 146, 363–370. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]


| Sample | xw | TP 1 | Hes 2 | Nat 3 | TC 4 | |
|---|---|---|---|---|---|---|
| OJC | 88.99 ± 0.05 b | 986 ± 92 a | 2681 ± 37 a | 288 ± 20 a | 28 ± 4 b | |
| OJC-FD | BD | 4.41 ± 0.07 a | 1024 ± 16 b | 4403 ± 50 b | 351 ± 7 b | 17.8 ± 0.4 a |
| AD | 157 ± 7 A | 16 ± 0 A | 7.9 ± 0.1 A | 0.9 ± 0.1 A | ||
| GA25 | BD | 3.42 ± 0.08 a | 1187 ± 10 c | 5325 ± 31 c | 370 ± 2 b | 16.7 ± 0.6 a |
| AD | 238 ± 7 B | 20.7 ± 0.1 B | 12.4 ± 0.1 B | 0.9 ± 0.9 A | ||
| OSA25 | BD | 3.46 ± 0.07 a | 1145 ± 7 c | 5548 ± 15 c | 405 ± 7 c | 14.0 ± 0.2 a |
| AD | 238 ± 6 B | 22.9 ± 0.1 C | 13.1 ± 0.1 B | 1.1 ± 0.6 B | ||
| GA45 | BD | 3.32 ± 0.07 a | 1369 ± 78 d | 6533 ± 46 d | 520 ± 27 d | 14.3 ± 0.3 a |
| AD | 323 ± 2 C | 26.6 ± 0.1 D | 16.3 ± 0.1 C | 1.27 ± 0.7 C | ||
| OSA45 | BD | 3.53 ± 0.05 a | 1321 ± 87 d | 6318 ± 42 d | 458 ± 15 d | 14.9 ± 0.3 a |
| AD | 285 ± 3 D | 28.9 ± 0.1 E | 17.2 ± 0.1 D | 1.67 ± 0.6 D | ||
| Sample | OJC-FD | GA25 | OSA25 | GA45 | OSA45 |
|---|---|---|---|---|---|
| TP | 15.29 a | 20.09 b | 20.83 b | 23.61 c | 21.62 c |
| HES | 0.36 a | 0.39 b | 0.41 b | 0.41 b | 0.46 c |
| NAT | 2.26 a | 3.33 b | 3.24 b | 2.94 b | 3.77 c |
| TC | 5.00 a | 5.42 a | 7.79 b | 8.89 b | 11.16 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Martínez, E.; Camacho, M.d.M.; Martínez-Navarrete, N. In Vitro Bioaccessibility of Bioactive Compounds of Freeze-Dried Orange Juice Co-Product Formulated with Gum Arabic and Modified Starch. Molecules 2023, 28, 810. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020810
García-Martínez E, Camacho MdM, Martínez-Navarrete N. In Vitro Bioaccessibility of Bioactive Compounds of Freeze-Dried Orange Juice Co-Product Formulated with Gum Arabic and Modified Starch. Molecules. 2023; 28(2):810. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020810
Chicago/Turabian StyleGarcía-Martínez, Eva, María del Mar Camacho, and Nuria Martínez-Navarrete. 2023. "In Vitro Bioaccessibility of Bioactive Compounds of Freeze-Dried Orange Juice Co-Product Formulated with Gum Arabic and Modified Starch" Molecules 28, no. 2: 810. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020810