A Comparative UHPLC-Q-Trap-MS/MS-Based Metabolomics Analysis to Distinguish Foeniculum vulgare Cultivars’ Antioxidant Extracts
Abstract
:1. Introduction
2. Results and Discussion
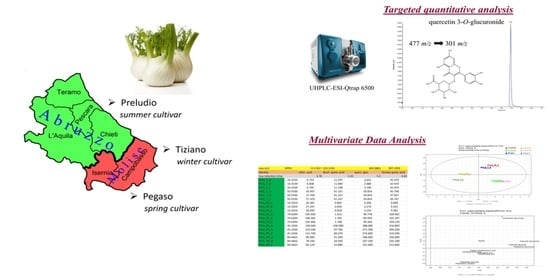
2.1. UPLC-ESI-QTRAP-MS/MS Quantitative Analysis
2.2. Method Validation
2.3. Antioxidant Activity and Content of Flavonoid
2.4. Multivariate Data Analysis
3. Materials and Methods
3.1. Raw Materials
3.2. Chemicals
3.3. Sample Preparation
3.4. Quantitative Analysis
3.4.1. ESI-QTRAP-MS and ESI-QTRAP-MS/MS Analyses
3.4.2. UPLC–ESI-QTRAP-MS/MS Analyses in MRM (Multiple Reaction Monitoring) Mode
3.4.3. Method Validation
3.5. DPPH Radical Scavenging Activity
3.6. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
3.7. Total Flavonoid Assay
3.8. Multivariate Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed. Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferioli, F.; Giambanelli, E.; D’Antuono, L.F. Fennel (Foeniculum vulgare Mill. subsp. piperitum) florets, a traditional culinary spice in Italy: Evaluation of phenolics and volatiles in local populations, and comparison with the composition of other plant parts. J. Sci. Food Agric. 2017, 97, 5369–5380. [Google Scholar] [CrossRef] [PubMed]
- Jahan, A. Fennel (Foeniculum vulgare). Plants Med. Values 2019, 32. [Google Scholar]
- Islam, M.; Srivastava, A.; Kumar, S.; Verma, N. Chemical and pharmacological properties of Foeniculum vulgare Mill: A review. WJPLS 2022, 7, 48–56. [Google Scholar]
- Esquivel-Ferriño, P.; Favela, J.M.d.J.H.; Garza Gonzalez, E.; Torres, N.; Rios Gomez, Y.; Del, M.; Camacho-Corona, M.d.R. Antimycobacterial Activity of Constituents from Foeniculum vulgare Var. Dulce Grown in Mexico. Molecules 2012, 7, 8471–8482. [Google Scholar] [CrossRef]
- Yakut, H.I.; Koyuncu, E.; Cakir, U.; Tayman, C.; Koyuncu, İ.; Taskin Turkmenoglu, T.; Cakir, E.; Ozyazici, A.; Aydogan, S.; Zenciroglu, A. Preventative and therapeutic effects of fennel (Foeniculum vulgare) seed extracts against necrotizing enterocolitis. J. Food Biochem. 2020, 44, e13284. [Google Scholar] [CrossRef]
- Choi, E.M.; Hwang, J.K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 2004, 75, 557–565. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Smolarz, H.D.; Kocki, J. Apoptotic activities of ethanol extracts from some Apiaceae on human leukaemia cell lines. Fitoterapia 2008, 79, 487–497. [Google Scholar] [CrossRef]
- Tognolini, M.; Ballabeni, V.; Bertoni, S.; Bruni, R.; Impicciatore, M.; Barocelli, E. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol. Res. 2007, 56, 254–260. [Google Scholar] [CrossRef]
- Mohamad, R.H.; El-Bastawesy, A.M.; Abdel-Monem, M.G.; Noor, A.M.; Al-Mehdar, H.A.; Sharawy, S.M.; El-Merzabani, M.M. Antioxidant and anticarcinogenic effects of methanolic extract and volatile oil of fennel seeds (Foeniculum vulgare). J. Med. Food 2011, 14, 986–1001. [Google Scholar] [CrossRef]
- Kaur, B.; Rolta, R.; Salaria, D.; Kumar, B.; Fadare, O.A.; da Costa, R.A.; Ahmad, A.; Al-Rawi, M.B.A.; Raish, M.; Rather, I.A. An in silico Investigation to Explore Anti-Cancer Potential of Foeniculum vulgare Mill. Phytoconstituents for the Management of Human Breast Cancer. Molecules 2022, 27, 4077. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and characterization of phenolic compounds in fennel (Foeniculum vulgare) using liquid chromatography-negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M. Characterization of some Italian types of wild fennel (Foeniculum vulgare Mill.). J. Agric. Food Chem. 2001, 49, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. LC-ESI/LTQOrbitrap/MS Metabolomic Analysis of Fennel Waste (Foeniculum vulgare Mill.) as a Byproduct Rich in Bioactive Compounds. Foods 2021, 10, 1893. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. UPLC-ESI-QTRAP-MS/MS Analysis to Quantify Bioactive Compounds in Fennel (Foeniculum vulgare Mill.) Waste with Potential Anti-Inflammatory Activity. Metabolites 2022, 12, 701. [Google Scholar] [CrossRef]
- Sonia, N.S.; Chandran, M.; Geethalekshmi, P. Vegetable peels as natural antioxidants for processed foods—A review. Agric. Rev. 2016, 37, 35–41. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Tu, M.; Xu, X.; Yang, S.; Chen, D. Current sample preparation methods and analytical techniques for the determination of synthetic antioxidants in edible oils. J. Sep. Sci. 2022, 45, 3874–3886. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [Google Scholar] [CrossRef]
- Zhou, D.A.-O.X.; Luo, M.A.-O.; Shang, A.A.-O.X.; Mao, Q.A.-O.; Li, B.A.-O.; Gan, R.A.-O.; Li, H.A.-O. Antioxidant Food Components for the Prevention and Treatment of Cardiovascular Diseases: Effects, Mechanisms, and Clinical Studies. Oxidative Med. Cell. Longev. 2021, 2021, 6627355. [Google Scholar] [CrossRef]
- Castaldo, L.; Izzo, L.; De Pascale, S.; Narváez, A.; Rodriguez-Carrasco, Y.; Ritieni, A. Chemical Composition, in vitro Bioaccessibility and Antioxidant Activity of Polyphenolic Compounds from Nutraceutical Fennel Waste Extract. Molecules 2021, 26, 1968. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.A.; Camilo, C.J.; de Fátima Alves Nonato, C.; Rodrigues, F.F.G.; Menezes, I.R.A.; Ribeiro-Filho, J.; Xiao, J.; de Almeida Souza, M.M.; da Costa, J.G.M. Influence of seasonal variation on phenolic content and in vitro antioxidant activity of Secondatia floribunda A. DC. (Apocynaceae). Food Chem. 2020, 315, 126277. [Google Scholar] [CrossRef] [PubMed]
- Aoussar, N.; Rhallabi, N.; Ait Mhand, R.; Manzali, R.; Bouksaim, M.; Douira, A.; Mellouki, F. Seasonal variation of antioxidant activity and phenolic content of Pseudevernia furfuracea, Evernia prunastri and Ramalina farinacea from Morocco. J. Saudi Soc. Agric. Sci. 2020, 19, 1–6. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, W.; Li, X.; Pan, X. Seasonal variations of phenolic profiles and antioxidant activity of walnut (Juglans sigillata Dode) green husks. Int. J. Food Prop. 2017, 20, S2635–S2646. [Google Scholar] [CrossRef] [Green Version]
- Jorge, T.F.; Mata, A.T.; António, C.A.-O. Mass spectrometry as a quantitative tool in plant metabolomics. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150370. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.F.; Zhou, B.; Ressom, H.W. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. Trends Analyt. Chem. 2012, 32, 1–14. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. ICH Guideline Q2(R2) on Validation of Analytical Procedures. Available online: europa.eu (accessed on 3 March 2022).
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Olivares-Vicente, M.; Barrajon-Catalan, E.; Herranz-Lopez, M.; Segura-Carretero, A.; Joven, J.; Encinar, J.A.; Micol, V. Plant-Derived Polyphenols in Human Health: Biological Activity, Metabolites and Putative Molecular Targets. Curr. Drug Metab. 2018, 19, 351–369. [Google Scholar] [CrossRef] [Green Version]
- Masullo, M.; Cerulli, A.; Mari, A.; de Souza Santos, C.C.; Pizza, C.; Piacente, S. LC-MS profiling highlights hazelnut (Nocciola di Giffoni PGI) shells as a byproduct rich in antioxidant phenolics. Food Res. Int. 2017, 101, 180–187. [Google Scholar] [CrossRef]
- Kılınç, H.; Masullo, M.; D’Urso, G.; Karayildirim, T.; Alankus, O.; Piacente, S. Phytochemical investigation of Scabiosa sicula guided by a preliminary HPLC-ESIMS. Phytochemistry 2020, 174, 112350. [Google Scholar] [CrossRef]
- Dobravalskytė, D.; Venskutonis, P.R.; Talou, T. Antioxidant properties and essential oil composition of Calamintha grandiflora L. Food Chem. 2012, 135, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Pizza, C.; Piacente, S.; Montoro, P. Combination of LC–MS based metabolomics and antioxidant activityfor evaluation of bioactive compounds in Fragaria vesca leaves from Italy. J. Pharm. Biomed. Anal. 2018, 150, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; D’Urso, G.; Kowalczyk, A.; Tuberoso, C.I.G. LC-ESI/LTQ-Orbitrap-MS Based Metabolomics in Evaluation of Bitter Taste of Arbutus unedo Honey. Molecules 2021, 26, 2765. [Google Scholar] [CrossRef] [PubMed]





| Compounds | DP | EP | CE | CXP | PI | DI | |
|---|---|---|---|---|---|---|---|
| 1 | neochlorogenic acid | −60 | −4 | −24 | −17 | 353 | 191 |
| 2 | dicaffeoylquinic acid | −61 | −4 | −24 | −38 | 515 | 353 |
| 3 | quercetin 3-O-glucoside | −138 | −9 | −28 | −32 | 463 | 301 |
| 4 | feruloylquinic acid | −89 | −4 | −33 | −21 | 367 | 191 |
| 5 | quercetin 3-O-glucuronide | −52 | −8 | −35 | −30 | 477 | 301 |
| 6 | isorhamnetin 3-O-glucuronide | −52 | −8 | −35 | −30 | 491 | 315 |
| 7 | kaempferol 3-O-glucuronide | −59 | −8 | −35 | −30 | 461 | 285 |
| 8 | dicaffeoylquinic acid malonyl * | −61 | −4 | −24 | −38 | 601 | 395 |
| 9 | kaempferol 3-O-rutinoside ** | −81 | −4 | −35 | −30 | 593 | 285 |
| 10 | kaempferol 3-O-glucoside ** | −81 | −4 | −25 | −30 | 447 | 285 |
| Compounds | FVLS-T | FVLE-T | FVLS-PE | FVLE-PE | FVLS-PR | FVLE-PR | |
|---|---|---|---|---|---|---|---|
| 1 | neochlorogenic acid | 6.80 ± 0.06 | 17.10 ± 0.86 | 28.84 ± 0.65 | 192.67 ± 3.34 | 151.74 ± 2.50 | 82.12 ± 2.63 |
| 2 | dicaffeoylquinic acid | 11.15 ± 0.21 | 41.17 ± 0.00 | 0.82 ± 0.02 | 1.71 ± 0.11 | 89.37 ± 2.42 | 22.88 ± 3.52 |
| 3 | quercetin 3-O-glucoside | 3.16 ± 0.67 | 50.85 ± 0.00 | 3.24 ± 0.05 | 90.36 ± 0.59 | 264.60 ± 3.09 | 81.60 ± 6.25 |
| 4 | feruloylquinic acid | 42.47 ± 0.00 | 46.77 ± 1.20 | 9.58 ± 0.36 | 103.13 ± 2.09 | 310.50 ± 5.91 | 185.60 ± 1.28 |
| 5 | quercetin 3-O-glucuronide | 1.14 ± 0.11 | 163.76 ± 2.61 | 11.14 ± 0.23 | 427.88 ± 8.36 | 1611.00 ± 16.11 | 872.00 ± 4.67 |
| 6 | isorhamnetin 3-O-glucuronide | 0.89 ± 0.13 | 257.90 ± 4.23 | 9.98 ± 1.02 | 888.86 ± 10.76 | 12.29 ± 1.07 | 5.26 ± 0.70 |
| 7 | kaempferol 3-O-glucuronide | 0.14 ± 0.00 | 37.84 ± 1.28 | 19.42 ± 0.26 | 238.76 ± 6.69 | 562.50 ± 10.73 | 366.40 ± 5.40 |
| 8 | dicaffeoylquinic acid malonyl * | 19.98 ± 1.01 | 48.18 ± 4.65 | nd | 0.61 ± 0.00 | 147.97 ± 5.46 | 107.72 ± 2.49 |
| 9 | kaempferol 3-O-rutinoside ** | nd | nd | nd | nd | 16.92 ± 0.87 | 8.09 ± 1.13 |
| 10 | kaempferol 3-O-glucoside ** | nd | nd | nd | nd | 15.24 ± 1.69 | 6.64 ± 0.75 |
| F. vulgare Extracts | TEAC [mg/mL ± SD a] b | DPPH [IC50 (mg/mL) ± SD a] | Total Flavonoids [mg/g Plant Extract (in RE) ± SD a] c |
|---|---|---|---|
| FVLS-T | 0.375 ± 0.006 | 5.782 ± 0.001 | ND |
| FVLE-T | 0.823 ± 0.008 | 0.342 ± 0.002 | 0.206 ± 0.006 |
| FVLS-PE | 0.448 ± 0.006 | 3.280 ± 0.002 | 0.083 ± 0.001 |
| FVLE-PE | 1.676 ± 0.010 | 0.634 ± 0.006 | 1.331 ± 0.002 |
| FVLS-PR | 0.509 ± 0.004 | 1.109 ± 0.002 | 0.309 ± 0.001 |
| FVLE-PR | 1.501 ± 0.009 | 0.010 ± 0.002 | 1.497 ± 0.002 |
| quercetin 3-O-glucoside | 1.813 ± 0.007 | / | / |
| vitamin C | / | 0.270 ± 0.006 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. A Comparative UHPLC-Q-Trap-MS/MS-Based Metabolomics Analysis to Distinguish Foeniculum vulgare Cultivars’ Antioxidant Extracts. Molecules 2023, 28, 900. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020900
Crescenzi MA, D’Urso G, Piacente S, Montoro P. A Comparative UHPLC-Q-Trap-MS/MS-Based Metabolomics Analysis to Distinguish Foeniculum vulgare Cultivars’ Antioxidant Extracts. Molecules. 2023; 28(2):900. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020900
Chicago/Turabian StyleCrescenzi, Maria Assunta, Gilda D’Urso, Sonia Piacente, and Paola Montoro. 2023. "A Comparative UHPLC-Q-Trap-MS/MS-Based Metabolomics Analysis to Distinguish Foeniculum vulgare Cultivars’ Antioxidant Extracts" Molecules 28, no. 2: 900. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020900