Multi-F-Structured MOF Materials Enhance Nanogenerator Output Performance for Corrosion Protection of Metallic Materials
Abstract
:1. Introduction
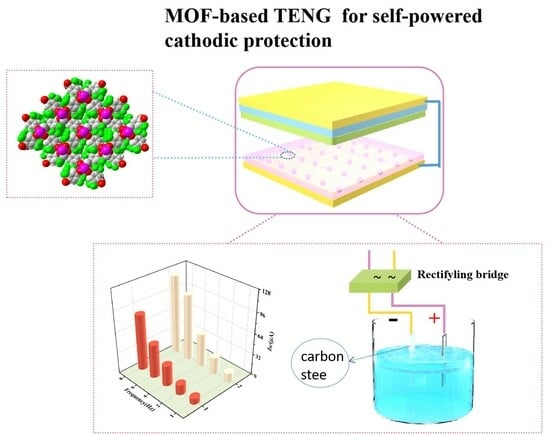
2. Results
3. Discussion
4. Materials and Methods
4.1. Compounds Preparation
4.2. Synthesis of [Co (2,3,5,6-Tetrafluoroterephthalic acid)] (Compound 1)
4.3. Synthesis of [Co (2,5-Bis(trifluoromethyl)terephthalic acid)] (Compound 2)
4.4. Preparation of 1/2 Base TENG
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Xiao, X.; Xu, P.; Zhao, T.; Song, L.; Pan, X.; Mi, J.; Xu, M.; Wang, Z.L. Dual-Tube Helmholtz Resonator-Based Triboelectric Nanogenerator for Highly Efficient Harvesting of Acoustic Energy. Adv. Energy Mater. 2019, 9, 1902824. [Google Scholar] [CrossRef]
- Zi, Y.; Wang, J.; Wang, S.; Li, S.; Wen, Z.; Guo, H.; Wang, Z.L. Effective energy storage from a triboelectric nanogenerator. Nat. Commun. 2016, 7, 10987. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Wang, Z.L. Triboelectric nanogenerators as flexible power sources. Npj Flex. Electron. 2017, 1, 10. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, C.; Kwon, S.H.; Jiang, Z.; Dong, L. Flexible Hybrid Piezoelectric-Electrostatic Device for Energy Harvesting and Sensing Applications. Adv. Mater. Interfaces 2023, 10, 2202173. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, S.; Huang, H.; Li, H.; Jia, B.; Ma, T. Solar Energy Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202204880. [Google Scholar] [CrossRef]
- Hayat, M.B.; Ali, D.; Monyake, K.C.; Alagha, L.; Ahmed, N. Solar energy-A look into power generation, challenges, and a solar-powered future. Int. J. Energy Res. 2019, 43, 1049–1067. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, A.C. On the origin of contact-electrification. Mater. Today 2019, 30, 34–51. [Google Scholar] [CrossRef]
- Wang, Z.L. From contact electrification to triboelectric nanogenerators. Rep. Prog. Phys. 2021, 84, 096502. [Google Scholar] [CrossRef]
- Wang, Z.L. On the expanded Maxwell’s equations for moving charged media system–General theory, mathematical solutions and applications in TENG. Mater. Today 2022, 52, 348–363. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric nanogenerators as new energy technology and self-powered sensors—Principles, problems and perspectives. Faraday Discuss 2014, 176, 447–458. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chen, J.; Lin, L. Progress in triboelectric nanogenerators as a new energy technology and self-powered sensors. Energy Environ. Sci. 2015, 8, 2250–2282. [Google Scholar] [CrossRef]
- Garcia, C.; Trendafilova, I. Real-time diagnosis of small energy impacts using a triboelectric nanosensor. Sens. Actuators A Phys. 2019, 291, 196–203. [Google Scholar] [CrossRef]
- Khandelwal, G.; Chandrasekhar, A.; Alluri, N.R.; Vivekananthan, V.; Maria Joseph Raj, N.P.; Kim, S.-J. Trash to energy: A facile, robust and cheap approach for mitigating environment pollutant using household triboelectric nanogenerator. Appl. Energy 2018, 219, 338–349. [Google Scholar] [CrossRef]
- Khandelwal, G.; Minocha, T.; Yadav, S.K.; Chandrasekhar, A.; Maria Joseph Raj, N.P.; Gupta, S.C.; Kim, S.-J. All edible materials derived biocompatible and biodegradable triboelectric nanogenerator. Nano Energy 2019, 65, 104016. [Google Scholar] [CrossRef]
- Papporello, R.L.; Miró, E.E.; Zamaro, J.M. Secondary growth of ZIF-8 films onto copper-based foils. Insight into surface interactions. Microporous Mesoporous Mater. 2015, 211, 64–72. [Google Scholar] [CrossRef]
- Fan, F.-R.; Lin, L.; Zhu, G.; Wu, W.; Zhang, R.; Wang, Z.L. Transparent Triboelectric Nanogenerators and Self-Powered Pressure Sensors Based on Micropatterned Plastic Films. Nano Lett. 2012, 12, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Tcho, I.-W.; Kim, W.-G.; Jeon, S.-B.; Park, S.-J.; Lee, B.J.; Bae, H.-K.; Kim, D.; Choi, Y.-K. Surface structural analysis of a friction layer for a triboelectric nanogenerator. Nano Energy 2017, 42, 34–42. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Bigdeli, F.; Lollar, C.T.; Morsali, A.; Zhou, H.C. Switching in Metal-Organic Frameworks. Angew. Chem. Int. Ed. Engl. 2020, 59, 4652–4669. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, N.; Zhang, J.; Yan, R.; Li, J.; Wang, L.; Wang, N.; Lv, M.; Zhang, M. Ultrasensitive aptamer-based protein assays based on one-dimensional core-shell nanozymes. Biosens. Bioelectron. 2020, 150, 111881. [Google Scholar] [CrossRef]
- Bonneau, M.; Lavenn, C.; Ginet, P.; Otake, K.-i.; Kitagawa, S. Upscale synthesis of a binary pillared layered MOF for hydrocarbon gas storage and separation. Green Chem. 2020, 22, 718–724. [Google Scholar] [CrossRef]
- Petit, C. Present and future of MOF research in the field of adsorption and molecular separation. Curr. Opin. Chem. Eng. 2018, 20, 132–142. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, P.-F.; Yang, G.-P.; Hou, L.; Zhang, W.-Y.; Han, Y.-F.; Liu, P.; Wang, Y.-Y. Supramolecular control of MOF pore properties for the tailored guest adsorption/separation applications. Coord. Chem. Rev. 2021, 434, 213709. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Kanchanakungwankul, S.; Lu, Z.; Noh, H.; Syed, Z.H.; Farha, O.K.; Truhlar, D.G.; Hupp, J.T. Unexpected “Spontaneous” Evolution of Catalytic, MOF-Supported Single Cu(II) Cations to Catalytic, MOF-Supported Cu(0) Nanoparticles. J. Am. Chem. Soc. 2020, 142, 21169–21177. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-P.; Gagliardi, L.; Truhlar, D.G. Cerium Metal-Organic Framework for Photocatalysis. J. Am. Chem. Soc. 2018, 140, 7904–7912. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Y.M.; Guo, W.; Yin, X.B. Color-tunable lanthanide metal-organic framework gels. Chem. Sci. 2019, 10, 1644–1650. [Google Scholar] [CrossRef]
- Zhou, Z.; Mukherjee, S.; Hou, S.; Li, W.; Elsner, M.; Fischer, R.A. Porphyrinic MOF Film for Multifaceted Electrochemical Sensing. Angew. Chem. Int. Ed. 2021, 60, 20551–20557. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, B.; Long, J.; Kuang, Y.; Chen, X.; Hou, M.; Gao, J.; Zhou, S.; Fan, B.; He, Y.; et al. Interfacial Laser-Induced Graphene Enabling High-Performance Liquid-Solid Triboelectric Nanogenerator. Adv. Mater. 2021, 33, e2104290. [Google Scholar] [CrossRef]
- Hao, C.; Wu, X.; Sun, M.; Zhang, H.; Yuan, A.; Xu, L.; Xu, C.; Kuang, H. Chiral Core–Shell Upconversion Nanoparticle@MOF Nanoassemblies for Quantification and Bioimaging of Reactive Oxygen Species in Vivo. J. Am. Chem. Soc. 2019, 141, 19373–19378. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.M.; Li, Y.H.; Cai, S.J.; Yin, X.B.; He, X.W.; Zhang, Y.K. Fluorescent Imaging-Guided Chemotherapy-and-Photodynamic Dual Therapy with Nanoscale Porphyrin Metal-Organic Framework. Small 2017, 13, 1603459. [Google Scholar] [CrossRef]
- Luo, T.Y.; Das, P.; White, D.L.; Liu, C.; Star, A.; Rosi, N.L. Luminescence “Turn-On” Detection of Gossypol Using Ln(3+)-Based Metal-Organic Frameworks and Ln(3+) Salts. J. Am. Chem. Soc. 2020, 142, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xin, N.; Qiao, Z.; Chen, S.; Zeng, L.; Zhang, Y.; Wei, D.; Sun, J.; Fan, H. Bioactive MOFs Based Theranostic Agent for Highly Effective Combination of Multimodal Imaging and Chemo-Phototherapy. Adv. Healthc. Mater. 2020, 9, 2000205. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wang, H.; Wang, C.; Liu, Z.; Li, K.; Zhu, Y. A sustainable dynamic redox reaction passive film for long-term anti-corrosion of carbon steel surface. J. Colloid Interface Sci. 2020, 580, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Berrissoul, A.; Loukili, E.; Mechbal, N.; Benhiba, F.; Guenbour, A.; Dikici, B.; Zarrouk, A.; Dafali, A. Anticorrosion effect of a green sustainable inhibitor on mild steel in hydrochloric acid. J. Colloid Interface Sci. 2020, 580, 740–752. [Google Scholar] [CrossRef]
- Zhu, E.; Liu, Q.; Yu, L.; He, M.; Chen, Q.; Chen, Z.; Chen, S.; Zhang, Z. Synthesis, Crystal Structure, and Electrochemical Property of the Complex [Co(tfbdc)(DMF)2(H2O)2] (tfbdc = Tetrafluoroterephthalate; DMF = N,N-Dimethylformamide). Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2011, 41, 1299–1304. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, J.-B.; Zhou, Y.-J.; Wang, S.-H.; Xiong, Z.-Q.; Zhang, Z.-K.; Zhang, S.-S.; Zhang, C.-K.; Xu, C.-F.; Liu, G.-Q. Multi-F-Structured MOF Materials Enhance Nanogenerator Output Performance for Corrosion Protection of Metallic Materials. Molecules 2023, 28, 7894. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28237894
Xiong J-B, Zhou Y-J, Wang S-H, Xiong Z-Q, Zhang Z-K, Zhang S-S, Zhang C-K, Xu C-F, Liu G-Q. Multi-F-Structured MOF Materials Enhance Nanogenerator Output Performance for Corrosion Protection of Metallic Materials. Molecules. 2023; 28(23):7894. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28237894
Chicago/Turabian StyleXiong, Jia-Bin, Yong-Juan Zhou, Shi-Hui Wang, Zhang-Qi Xiong, Zi-Kun Zhang, Shan-Shan Zhang, Chen-Kunlun Zhang, Chao-Fan Xu, and Guo-Qun Liu. 2023. "Multi-F-Structured MOF Materials Enhance Nanogenerator Output Performance for Corrosion Protection of Metallic Materials" Molecules 28, no. 23: 7894. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28237894