Recombinant Domain of Flagellin Promotes In Vitro a Chemotactic Inflammatory Profile in Human Immune Cells Independently of a Dendritic Cell Phenotype
Abstract
:1. Introduction
2. Results
2.1. rND1 Is Not Toxic and Promotes an In Vitro Proinflammatory Response in Human Immune Cells
2.2. rND1 Promotes an In Vitro Chemotactic Response in Human Immune Cells
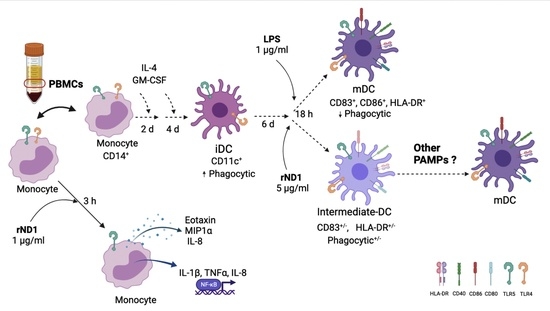
2.3. Monocyte- Derived Dendritic Cell (MoDC) Are Insensitive to rND1 Stimulation
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Expression and Purification of Recombinant D1 Domain
4.3. Isolation of Human Peripheral Blood Mononuclear Cells (PBMCs)
4.4. Cell Culture and Treatments
4.4.1. THP-1 Cell Line
4.4.2. PBMC
4.4.3. Monocytes-Derived Dendritic Cells (MoDC)
4.5. MTT Assay
4.6. Determination of Cytokines
4.7. Flow Cytometry Analysis of MoDCs
4.8. Phagocytosis Assay
4.9. RNA Extraction and cDNA Synthesis
4.10. Analysis of Gene Expression
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef]
- Moynagh, P.N. TLR signalling and activation of IRFs: Revisiting old friends from the NF-kappaB pathway. Trends Immunol. 2005, 26, 469–476. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D.; Andersen-Nissen, E.; Hayashi, F.; Strobe, K.; Bergman, M.A.; Barrett, S.L.; Cookson, B.T.; Aderem, A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 2003, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ahmed, J.; Wang, G.; Hassan, I.; Strulovici-Barel, Y.; Salit, J.; Mezey, J.G.; Crystal, R.G. Airway epithelial expression of TLR5 is downregulated in healthy smokers and smokers with chronic obstructive pulmonary disease. J. Immunol. 2012, 189, 2217–2225. [Google Scholar] [CrossRef] [Green Version]
- Narayanankutty, A.; Sasidharan, A.; Job, J.T. Targeting Toll like Receptors in Cancer: Role of TLR Natural and Synthetic Modulators. Curr. Pharm. Des. 2020, 26, 5040–5053. [Google Scholar] [CrossRef]
- Anwar, M.A.; Shah, M.; Kim, J.; Choi, S. Recent clinical trends in Toll-like receptor targeting therapeutics. Med. Res. Rev. 2019, 39, 1053–1090. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Song, L.; Reiserova, L.; Trivedi, U.; Li, H.; Liu, X.; Noah, D.; Hou, F.; Weaver, B.; Tussey, L. Flagellin-HA vaccines protect ferrets and mice against H5N1 highly pathogenic avian influenza virus (HPAIV) infections. Vaccine 2012, 30, 6833–6838. [Google Scholar] [CrossRef]
- Wang, B.Z.; Gill, H.S.; Kang, S.M.; Wang, L.; Wang, Y.C.; Vassilieva, E.V.; Compans, R.W. Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand. Clin. Vaccine Immunol. 2012, 19, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- McDonald, W.F.; Huleatt, J.W.; Foellmer, H.G.; Hewitt, D.; Tang, J.; Desai, P.; Price, A.; Jacobs, A.; Takahashi, V.N.; Huang, Y.; et al. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J. Infect. Dis. 2007, 195, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Bargieri, D.Y.; Rosa, D.S.; Braga, C.J.; Carvalho, B.O.; Costa, F.T.; Espíndola, N.M.; Vaz, A.J.; Soares, I.S.; Ferreira, L.C.; Rodrigues, M.M. New malaria vaccine candidates based on the Plasmodium vivax Merozoite Surface Protein-1 and the TLR-5 agonist Salmonella Typhimurium FliC flagellin. Vaccine 2008, 26, 6132–6142. [Google Scholar] [CrossRef]
- Hamzabegovic, F.; Goll, J.B.; Hooper, W.F.; Frey, S.; Gelber, C.E.; Abate, G. Flagellin adjuvanted F1/V subunit plague vaccine induces T cell and functional antibody responses with unique gene signatures. NPJ Vaccines 2020, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Liu, L.; Wen, K.; Huang, J.; Geng, S.; Shen, J.; Pan, Z.; Jiao, X. Chimeric flagellin expressed by Salmonella typhimurium induces an ESAT-6-specific Th1-type immune response and CTL effects following intranasal immunization. Cell. Mol. Immunol. 2011, 8, 496–501. [Google Scholar] [CrossRef]
- Hajam, I.A.; Dar, P.A.; Shahnawaz, I.; Jaume, J.C.; Lee, J.H. Bacterial flagellin-a potent immunomodulatory agent. Exp. Mol. Med. 2017, 49, e373. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.T.; Hong, S.H.; Sin, J.I.; Vu, H.V.; Jeong, K.; Cho, K.O.; Uematsu, S.; Akira, S.; Lee, S.E.; Rhee, J.H. Flagellin enhances tumor-specific CD8⁺ T cell immune responses through TLR5 stimulation in a therapeutic cancer vaccine model. Vaccine 2013, 31, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Hong, S.H.; Rhee, J.H.; Lee, S.E. Optimal long peptide for flagellin-adjuvanted HPV E7 cancer vaccine to enhance tumor suppression in combination with anti-PD-1. Transl. Cancer Res. 2022, 11, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Cherukula, K.; Bang, Y.J.; Vijayan, V.; Moon, M.J.; Thiruppathi, J.; Puth, S.; Jeong, Y.Y.; Park, I.K.; Lee, S.E.; et al. Combination of Photodynamic Therapy and a Flagellin-Adjuvanted Cancer Vaccine Potentiated the Anti-PD-1-Mediated Melanoma Suppression. Cells 2020, 9, 2432. [Google Scholar] [CrossRef]
- Dong, B.; Wang, L.; Nie, S.; Li, X.; Xiao, Y.; Yang, L.; Meng, X.; Zhao, P.; Cui, C.; Tu, L.; et al. Anti-glioma effect of intracranial vaccination with tumor cell lysate plus flagellin in mice. Vaccine 2018, 36, 8148–8157. [Google Scholar] [CrossRef]
- Cai, Z.; Sanchez, A.; Shi, Z.; Zhang, T.; Liu, M.; Zhang, D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 2011, 71, 2466–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdelya, L.G.; Brackett, C.M.; Kojouharov, B.; Gitlin, I.I.; Leonova, K.I.; Gleiberman, A.S.; Aygun-Sunar, S.; Veith, J.; Johnson, C.; Haderski, G.J.; et al. Central role of liver in anticancer and radioprotective activities of Toll-like receptor 5 agonist. Proc. Natl. Acad. Sci. USA 2013, 110, E1857–E1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.E.; Hong, S.H.; Verma, V.; Lee, Y.S.; Duong, T.N.; Jeong, K.; Uthaman, S.; Sung, Y.C.; Lee, J.T.; Park, I.K.; et al. Flagellin is a strong vaginal adjuvant of a therapeutic vaccine for genital cancer. Oncoimmunology 2015, 5, e1081328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toshkov, I.A.; Gleiberman, A.S.; Mett, V.L.; Hutson, A.D.; Singh, A.K.; Gudkov, A.V.; Burdelya, L.G. Mitigation of Radiation-Induced Epithelial Damage by the TLR5 Agonist Entolimod in a Mouse Model of Fractionated Head and Neck Irradiation. Radiat. Res. 2017, 187, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Seed, T.M. Entolimod as a radiation countermeasure for acute radiation syndrome. Drug Discov. Today 2021, 26, 17–30. [Google Scholar] [CrossRef]
- González-Stegmaier, R.; Romero, A.; Estepa, A.; Montero, J.; Mulero, V.; Mercado, L. Effects of recombinant flagellin B and its ND1 domain from Vibrio anguillarum on macrophages from gilthead seabream (Sparus aurata L.) and rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish. Immunol. 2015, 42, 144–152. [Google Scholar] [CrossRef] [PubMed]
- González-Stegmaier, R.; Peña, A.; Villarroel-Espíndola, F.; Aguila, P.; Oliver, C.; MacLeod-Carey, D.; Rozas-Serri, M.; Enriquez, R.; Figueroa, J. Full recombinant flagellin B from Vibrio anguillarum (rFLA) and its recombinant D1 domain (rND1) promote a pro-inflammatory state and improve vaccination against P. salmonis in Atlantic salmon (S. salar). Dev. Comp. Immunol. 2021, 117, 103988. [Google Scholar] [CrossRef] [PubMed]
- Song, W.S.; Jeon, Y.J.; Namgung, B.; Hong, M.; Yoon, S.I. A conserved TLR5 binding and activation hot spot on flagellin. Sci. Rep. 2017, 7, 40878. [Google Scholar] [CrossRef] [Green Version]
- Eaves-Pyles, T.D.; Wong, H.R.; Odoms, K.; Pyles, R.B. Salmonella flagellin-dependent proinflammatory responses are localized to the conserved amino and carboxyl regions of the protein. J. Immunol. 2001, 167, 7009–7016. [Google Scholar] [CrossRef] [Green Version]
- Eaves-Pyles, T.; Bu, H.F.; Tan, X.D.; Cong, Y.; Patel, J.; Davey, R.A.; Strasser, J.E. Luminal-applied flagellin is internalized by polarized intestinal epithelial cells and elicits immune responses via the TLR5 dependent mechanism. PLoS ONE 2011, 6, e24869. [Google Scholar] [CrossRef]
- Xu, M.; Xie, Y.; Tan, M.; Zheng, K.; Xiao, Y.; Jiang, C.; Zhao, F.; Zeng, T.; Wu, Y. The N-terminal D1 domain of Treponema pallidum flagellin binding to TLR5 is required but not sufficient in activation of TLR5. J. Cell. Mol. Med. 2019, 23, 7490–7504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen-Nissen, E.; Smith, K.D.; Bonneau, R.; Strong, R.K.; Aderem, A. A conserved surface on Toll-like receptor 5 recognizes bacterial flagellin. J. Exp. Med. 2007, 204, 393–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen-Nissen, E.; Smith, K.D.; Strobe, K.L.; Barrett, S.L.; Cookson, B.T.; Logan, S.M.; Aderem, A. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. USA. 2005, 102, 9247–9252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediat. Inflamm. 2013, 2013, 697972. [Google Scholar] [CrossRef] [Green Version]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shortman, K.; Liu, Y.J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002, 2, 151–161. [Google Scholar] [CrossRef]
- Dowling, D.; Hamilton, C.M.; O’Neill, S.M. A comparative analysis of cytokine responses, cell surface marker expression and MAPKs in DCs matured with LPS compared with a panel of TLR ligands. Cytokine 2008, 41, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, J. Properties of immature and mature dendritic cells: Phenotype, morphology, phagocytosis, and migration. RSC Adv. 2019, 9, 11230–11238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, C.Y.; Kim, S.Y.; Lee, H.J.; Lee, S.E.; Lim, S.C.; Rhee, J.H.; Lee, J.J. A bacterial flagellin in combination with proinflammatory cytokines activates human monocyte-derived dendritic cells to generate cytotoxic T lymphocytes having increased homing signals to cancer. J. Immunother. 2014, 37, 16–25. [Google Scholar] [CrossRef]
- Means, T.K.; Hayashi, F.; Smith, K.D.; Aderem, A.; Luster, A.D. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 2003, 170, 5165–5175. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, S.; Tahk, S.; Lohner, A.; Hänel, G.; Maiser, A.; Hauke, M.; Patel, L.; Rothe, M.; Josenhans, C.; Leonhardt, H.; et al. Fusion of Bacterial Flagellin to a Dendritic Cell-Targeting αCD40 Antibody Construct Coupled With Viral or Leukemia-Specific Antigens Enhances Dendritic Cell Maturation and Activates Peptide-Responsive T Cells. Front. Immunol. 2020, 11, 602802. [Google Scholar] [CrossRef]
- Bustin, S.A. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Stegmaier, R.; Aguirre, A.; Cárcamo, C.; Aguila-Torres, P.; Villarroel-Espíndola, F. Recombinant Domain of Flagellin Promotes In Vitro a Chemotactic Inflammatory Profile in Human Immune Cells Independently of a Dendritic Cell Phenotype. Molecules 2023, 28, 2394. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28052394
González-Stegmaier R, Aguirre A, Cárcamo C, Aguila-Torres P, Villarroel-Espíndola F. Recombinant Domain of Flagellin Promotes In Vitro a Chemotactic Inflammatory Profile in Human Immune Cells Independently of a Dendritic Cell Phenotype. Molecules. 2023; 28(5):2394. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28052394
Chicago/Turabian StyleGonzález-Stegmaier, Roxana, Adam Aguirre, Constanza Cárcamo, Patricia Aguila-Torres, and Franz Villarroel-Espíndola. 2023. "Recombinant Domain of Flagellin Promotes In Vitro a Chemotactic Inflammatory Profile in Human Immune Cells Independently of a Dendritic Cell Phenotype" Molecules 28, no. 5: 2394. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28052394