Analysis of the Free Amino Acid Profile of Barley Grain from Organic Fertilisation with Ash from Biomass Combustion
Abstract
:1. Introduction
2. Results
2.1. Total Protein Content in Grain
2.2. Content of Total Amino Acids in Barley Flour
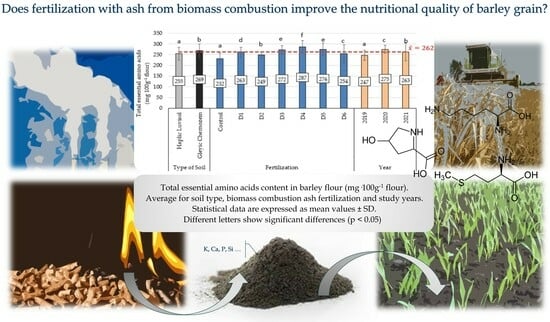
2.3. Content of Total Essential Amino Acids in Barley Flour
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Field Experiment
- -
- BBCH 31-33: Aviator Xpro 225EC (prothioconazole + bixafen; 0.8 dm3·ha−1) + Moddus 250EC (trinexapac-ethyl; 0.35 dm3·ha−1) + Karate Zeon 050 CS (lambda-cyhalothrin; 0.075 dm3·ha−1) + Isotak Max (adjuvant; 0.2 dm3·ha−1);
- -
- BBCH 31-33: Granstar 75WG (tribenuron-methyl; 20 g·kg−1) + Alfa 100EC (alpha-cypermethrin; 0.1 dm3·ha−1) + Prosupero (adjuvant; 0.5 dm3·ha−1);
- -
- BBCH 35-39: Ephon Top (ethephon; 0.75 dm3·ha−1) + Amistar 250 S.C. (azoxystrobin; 0.4 dm3·ha−1) + Aviator Xpro 225EC (prothioconazole + bixafen; 0.3 dm3·ha−1) + Alfa 100EC (alpha-cypermethrin; 0.1 dm3·ha−1) + Prosupero (adjuvant; 0.3 dm3·ha−1);
- -
- BBCH 51-55: Amistar 250 S.C. (azoxystrobin; 0.3) + Helicur 250 EW (tebuconazole; 0.7) + Karate Zeon 050 CS (lambda-cyhalothrin; 0.075) + Isotak Max (adjuvant; 0.2 dm3·ha−1).
4.3. Analytical Procedures
4.3.1. Determination of Protein Content
4.3.2. Assay of Free Amino Acid Profiles
4.4. Chemicals and Reagents
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hager, A.S.; Taylor, J.P.; Waters, D.M.; Arendt, E.K. Gluten free beer—A review. Trends Food Sci. Technol. 2014, 36, 44–54. [Google Scholar] [CrossRef]
- Howitt, C.A.; Larkin, P.J.; Colgrave, M.L. Gluten reduction strategies for wheat and barley. Cereal Food World 2018, 65, 184–187. [Google Scholar] [CrossRef]
- Syu, K.Y.; Lin, C.L.; Huang, H.C.; Lin, J.K. Determination of theanine, GABA, and other amino acids in green, oolong, black, and Pu-erh teas with dabsylation and high-performance liquid chromatography. J. Agric. Food Chem. 2008, 56, 7637–7643. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.-Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Jefferson, L.S. New functions for amino acids: Effects on gene transcription and translation. Am. J. Clin. Nutr. 2006, 83, 500S–507S. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Pinto, M. Tea: A new perspective on health benefits. Food Res. Int. 2013, 53, 558–567. [Google Scholar] [CrossRef]
- Bottecchia, C.; Noël, T. Photocatalytic modification of amino acids, peptides, and proteins. Chem. Eur. J. 2019, 25, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef]
- FAO/WHO. Codex Alimentarius Commission Annex Proposed Standard for Fermented Milks (A11) CL 1997—MMP; FAO: Rome, Italy, 1997. [Google Scholar]
- Walther, B.; Sieber, R. Bioactive proteins and peptides in foods. Int. J. Vitam. Nutr. Res. 2011, 81, 181–192. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie/Nutrition Standards for the Polish Population and Their Application; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny/National Institute of Public Health—National Institute of Hygiene Zakład Higieny: Warsaw, Poland, 2020; 83p, ISBN 978-83-65870-28-5. Available online: https://www.pzh.gov.pl/wp-content/uploads/2020/12/Normy_zywienia_2020web-1.pdf (accessed on 20 November 2023). (In Polish)
- Iwaniuk, P.; Konecki, R.; Kaczynski, P.; Rysbekova, A.; Lozowicka, B. Influence of seven levels of chemical/biostimulator protection on amino acid profile and yield traits in wheat. Crop J. 2022, 10, 1198–1206. [Google Scholar] [CrossRef]
- Gorash, O.; Klymyshena, R.; Zinchenko, O.; Strilets, O. Influence of foliar fertilization with micro-fertilizers on physiological grain quality of spring malting barley. Ukr. J. Ecol. 2021, 11, 15–20. [Google Scholar] [CrossRef]
- Tehulie, N.S.; Eskezia, H. Effects of nitrogen fertilizer rates on growth, yield components and yield of food Barley (Hordeum vulgare L.): A Review. J. Plant Sci. Agric. Res. 2021, 5, 46. [Google Scholar]
- Pycia, K.; Juszczak, L.; Gałkowska, D.; Witczak, M. Physicochemical properties of starches obtained from Polish potato cultivars. Starch/Stärke 2012, 64, 105–114. [Google Scholar] [CrossRef]
- Liszka-Skoczylas, M. Wpływ nawożenia roślin ziemniaka (Solanum tuberosum L.) na zawartość i jakość skrobi w bulwach. ŻYWNOŚĆ. Nauka. Technologia. Jakość 2020, 27, 31–46. [Google Scholar] [CrossRef]
- Cruz-Paredes, C.; López-García, Á.; Rubæk, G.H.; Hovmand, M.F.; Sørensen, P.; Kjøller, R. Risk assessment of replacing conventional P fertilizers with biomass ash: Residual effects on plant yield, nutrition, cadmium accumulation and mycorrhizal status. Sci. Total Environ. 2017, 575, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rubæk, G.H.; Sørensen, P. High plant availability of phosphorus and low availability of cadmium in four biomass combustion ashes. Sci. Total Environ. 2016, 557–558, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Iderawumi, A.M. Effects of ash on soil properties and yield of crops. Agric. Obs. 2020, 1, 8. Available online: https://www.researchgate.net/publication/344000712_Effects_of_Ash_on_Soil_Properties_and_Yield_of_Crops (accessed on 20 November 2023).
- Pels, J.R.; de Nie, D.S.; Kiel, J.H. Utilization of ashes from biomass combustion and gasification. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005; Volume 17, pp. 17–21. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Qin, J.; Hovmand, M.F.; Ekelund, F.; Rønn, R.; Christensen, S.; de Groot, G.A.; Mortensen, L.H.; Skov, S.; Krogh, P.H. Wood ash application increases pH but does not harm the soil mesofauna. Environ. Pollut. 2017, 224, 581–589. [Google Scholar] [CrossRef]
- Panagos, P.; Ballabio, C.; Poesen, J.; Lugato, E.; Scarpa, S.; Montanarella, L.; Borrelli, P. A soil erosion indicator for supporting agricultural, environmental and climate policies in the European Union. Remote Sens. 2020, 12, 1365. [Google Scholar] [CrossRef]
- Binner, H.; Sullivan, T.; Jansen, M.A.K.; McNamara, M.E. Metals in urban soils of Europe: A systematic review. Sci. Total Environ. 2023, 854, 158734. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J. A preliminary investigation on determining the “available” phosphorus in Saskatchewan soils. Sci. Agric. 1932, 12, 346–351. [Google Scholar]
- McKenzie, R.H.; Bremer, E.; Kryzanowski, L.; Middleton, A.B.; Solberg, E.D.; Heaney, D.; Coy, G.; Harapiak, J. Yield benefit of phosphorus fertilizer for wheat, barley and canola in Alberta. Can. J. Soil Sci. 2003, 83, 431–441. [Google Scholar] [CrossRef]
- Eppendorfer, W.H.; Bille, S.W. Free and total amino acid composition of edible parts of beans, kale, spinach, cauliflower and potatoes as influenced by nitrogen fertilisation and phosphorus and potassium deficiency. J. Sci. Food Agric. 1996, 71, 449–458. [Google Scholar] [CrossRef]
- Stadnik, B.; Tobiasz-Salach, R.; Mazurek, M. Physiological and epigenetic reaction of barley (Hordeum vulgare L.) to the foliar application of silicon under soil salinity conditions. Int. J. Mol. Sci. 2022, 23, 1149. [Google Scholar] [CrossRef] [PubMed]
- Szostek, M.; Szpunar-Krok, E.; Ilek, A. Chemical speciation of trace elements in soil fertilized with biomass combustion ash and their accumulation in winter oilseed rape plants. Agronomy 2023, 13, 942. [Google Scholar] [CrossRef]
- Tobiasz-Salach, R.; Augustyńska-Prejsnar, A. Response of spring barley to foliar fertilization with Cu and Mn. Acta Sci. Pol. Agric. 2020, 19, 29. [Google Scholar] [CrossRef]
- Tigre, W.; Worku, W.; Haile, W. Effects of nitrogen and phosphorus fertilizer levels on growth and development of barley (Hordeum vulgare L.) at Bore District, Southern Oromia, Ethiopia. Am. J. Life Sci. 2014, 2, 260–266. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Szostek, M.; Pawlak, R.; Gorzelany, J.; Migut, D. Effect of fertilisation with ash from biomass combustion on the mechanical properties of potato tubers (Solanum tuberosum L.) grown in two types of soil. Agronomy 2022, 12, 379. [Google Scholar] [CrossRef]
- Jenkins, B.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Szostek, M.; Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Ilek, A. Short-term effect of fly ash from biomass combustion on spring rape plants growth, elements accumulation, and soil properties. Int. J. Environ. Res. Public Health. 2023, 20, 455. [Google Scholar] [CrossRef]
- Silva, F.C.; Cruz, N.C.; Tarelho, L.A.; Rodrigues, S.M. Use of biomass ash-based materials as soil fertilizers: Critical review of the existing regulatory framework. J. Clean. Prod. 2019, 214, 112–124. [Google Scholar] [CrossRef]
- Decision of the Minister of Agriculture and Rural Development, Poland No. G-1311/23 dated 01.03.2023. Case Sign: DHR.pn.8101.10.2023. Available online: https://www.gov.pl/web/rolnictwo/wykaz-nawozow-i-srodkow-wspomagajacych-uprawe-roslin (accessed on 20 November 2023).
- Pycia, K.; Szupnar-Krok, E.; Szostek, M.; Pawlak, R.; Juszczak, L. Effect of soil type and application of ecological fertilizer composed of ash from biomass combustion on selected physicochemical, thermal, and rheological properties of potato starch. Molecules 2022, 27, 4318. [Google Scholar] [CrossRef] [PubMed]
- Chavan, J.K.; Kadam, S.S.; Beuchat, L.R. Nutritional improvement of cereals by sprouting. Crit. Rev. Food Sci. Nutr. 1989, 28, 401–437. [Google Scholar] [CrossRef] [PubMed]
- Miralles, D.J.; Abeledo, L.G.; Prado, S.A.; Chenu, K.; Serrago, R.A.; Savin, R. Barley. In Crop Physiology Case Histories for Major Crops; Academic Press: Cambridge, MA, USA, 2021; pp. 164–195. [Google Scholar]
- Assween, M. Amino acid composition of spring barley cultivars used in Norway. Acta Agric. Scand. B Soil Plant Sci. 2009, 59, 395–401. [Google Scholar] [CrossRef]
- Jaeger, A.; Zannini, E.; Sahin, A.W.; Arendt, E.K. Barley protein properties, extraction and applications, with a focus on brewers’ spent grain protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Koller, H.; Perkins, L.B. Brewing and the chemical composition of amine-containing compounds in beer: A review. Foods 2022, 11, 257. [Google Scholar] [CrossRef]
- Knežević, D.; Đukić, N.; Madic, M.; Paunović, A.; Zecevic, V. Comparison of amino acids contents in barley and wheat. Res. J. Agric. Sci. 2007, 39, 71–76. Available online: https://rivec.institut-palanka.rs/bitstream/handle/123456789/379/bitstream_973.pdf?sequence=1&isAllowed=y (accessed on 20 November 2023).
- Singh, T.; Sosulski, F.W. Amino acid composition of malts: Effect of germination and gibberellic acid on hulled and hulless barley and utility wheat. J. Agric. Food Chem. 1986, 34, 1012–1016. [Google Scholar] [CrossRef]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, Protein and mineral fortification of wheat bread through milled and fermented brewer’s spent grain enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- Yilmaz, S.; İlbaş, A.İ.; Akbulut, M.; Çetin, A. Grain amino acid composition of barley (Hordeum vulgare L.) cultivars subjected to selenium doses. Turk. J. Biochem. 2018, 43, 268–276. [Google Scholar] [CrossRef]
- Beljkaš, B.; Matić, J.; Milovanović, I.; Javanov, P.; Mišan, A.; Šarić, L. Rapid method for determination of protein content in cereals and oilseeds: Validation, measurement uncertainty and comparison with the Kjeldahl method. Accredit. Qual. Assur. 2010, 15, 555–561. [Google Scholar] [CrossRef]
- Tarapatskyy, M.; Zaguła, G.; Bajcar, M.; Puchalski, C.; Saletnik, B. Magnetic field extraction techniques in preparing high-quality tea infusions. Appl. Sci. 2018, 8, 1876. [Google Scholar] [CrossRef]
- Pęksa, A.; Miedzianka, J.; Nemś, A.; Rytel, E. The Free-Amino-Acid Content in Six Potatoes Cultivars through Storage. Molecules 2021, 26, 1322. [Google Scholar] [CrossRef]




| Type of Soil | Fertilisation | Taurine (TAU) | Gamma-Aminobutyric Acid (GABA) |
|---|---|---|---|
| mg·100 g−1 Flour | |||
| Haplic Luvisol | Control | 10.8 ± 3.0 e | 30.6 ± 4.6 cd |
| D1 | 9.9 ± 3.5 b | 37.9 ± 12.7 i | |
| D2 | 12.9 ± 3.3 h | 32.3 ± 4.9 f | |
| D3 | 13.1 ± 3.6 h | 39.5 ± 14.8 j | |
| D4 | 13.6 ± 4.5 ij | 39.1 ± 12.3 j | |
| D5 | 10.6 ± 1.8 de | 33.2 ± 8.2 g | |
| D6 | 9.5 ± 1.6 a | 28.6 ± 4.0 ab | |
| Gleyic Chernozem | Control | 10.4 ± 2.4 cd | 29.1 ± 2.9 b |
| D1 | 14.2 ± 5.3 k | 29.9 ± 0.6 c | |
| D2 | 10.3 ± 1.7 c | 28.9 ± 0.8 b | |
| D3 | 11.6 ± 3.3 f | 31.1 ± 2.9 de | |
| D4 | 13.9 ± 5.4 j | 34.4 ± 2.8 h | |
| D5 | 13.4 ± 4.6 i | 31.5 ± 2.3 e | |
| D6 | 12.1 ± 3.5 g | 27.9 ± 3.8 a | |
| Mean for factors | |||
| Type of Soil (S) | Haplic Luvisol | 11.5 ± 3.4 a | 34.5 ± 10.1 b |
| Gleyic Chernozem | 12.3 ± 4.1 b | 30.4 ± 3.2 a | |
| Fertilisation (F) | Control | 10.6 ± 2.6 a | 29.9 ± 3.8 b |
| D1 | 12.1 ± 4.9 d | 33.9 ± 9.7 e | |
| D2 | 11.6 ± 2.9 c | 30.6 ± 3.9 c | |
| D3 | 12.3 ± 3.4 e | 35.3 ± 11.2 f | |
| D4 | 13.8 ± 4.9 f | 36.8 ± 9.0 g | |
| D5 | 12.0 ± 3.7 d | 32.3 ± 5.9 d | |
| D6 | 10.8 ± 2.9 b | 28.3 ± 3.8 a | |
| Year (Y) | 2019 | 7.8 ± 0.6 a | 38.0 ± 11.1 c |
| 2020 | 12.8 ± 2.3 b | 29.3 ± 2.7 a | |
| 2021 | 15.1 ± 2.9 c | 30.1 ± 1.9 b | |
| Two-way ANOVA (F/p value) | |||
| S | 632.0/0.000 | 2640.8/0.000 | |
| F | 634.4/0.000 | 858.9/0.000 | |
| Y | 18,723.9/0.000 | 4854.0/0.000 | |
| S*F | 891.7/0.000 | 222.6/0.000 | |
| S*Y | 290.0/0.000 | 6119.2/0.000 | |
| F*Y | 317.6/0.000 | 334.3/0.000 | |
| S*F*Y | 354.8/0.000 | 221.1/0.000 | |
| Type of Soil | Fertilisation | Aspartic Acid (ASP) | Serine (SER) | Asparagine (ASN) | Glutamic Acid (GLU) | Glycine (GLY) | Alanine (ALA) | Cysteine (CYS) | Tyrosine (TYR) | Ornithine (ORN) | Proline (PRO) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg·100 g−1 Flour | |||||||||||
| Haplic Luvisol | Control | 82.0 ± 8.0 e | 11.3 ± 7.5 a | 360 ± 61 c | 111 ± 7 c | 10.9 ± 3.0 b | 32.2 ± 14.1 b | 5.27 ± 4.61 i | 27.1 ± 9.2 b | 1.37 ± 0.38 c | 50.7 ± 27.9 e |
| D1 | 85.9 ± 6.2 f | 14.2 ± 10.3 d | 365 ± 47 cd | 118 ± 8 e | 10.4 ± 0.9 a | 36.4 ± 13.6 e | 3.86 ± 1.53 f | 30.0 ± 5.0 de | 1.19 ± 0.45 b | 64.2 ± 36.9 h | |
| D2 | 87.6 ± 10.7 f | 15.7 ± 13.0 f | 371 ± 48 d | 122 ± 20 f | 13.2 ± 4.6 g | 42.6 ± 23.0 h | 3.48 ± 2.20 d | 29.0 ± 8.5 cd | 2.40 ± 1.50 g | 62.4 ± 33.0 g | |
| D3 | 91.8 ± 11.7 g | 16.8 ± 14.0 h | 383 ± 43 e | 98 ± 28 a | 13.4 ± 4.8 gh | 45.3 ± 24.9 i | 4.91 ± 1.38 h | 31.2 ± 9.5 fg | 3.20 ± 1.65 i | 71.1 ± 56.6 k | |
| D4 | 92.5 ± 12.8 g | 16.1 ± 11.5 g | 400 ± 63 f | 134 ± 21 h | 14.2 ± 3.7 i | 47.5 ± 20.5 j | 5.05 ± 2.90 hi | 31.4 ± 11.4 fg | 2.35 ± 1.85 fg | 67.7 ± 48.3 j | |
| D5 | 86.1 ± 14.8 f | 16.9 ± 15.0 h | 344 ± 16 b | 117 ± 23 e | 12.8 ± 5.4 f | 43.3 ± 25.6 h | 3.61 ± 1.29 de | 30.6 ± 9.6 ef | 1.65 ± 0.84 d | 68.8 ± 42.2 j | |
| D6 | 82.2 ± 8.6 e | 14.0 ± 12.3 d | 338 ± 29 ab | 112 ± 19 c | 10.8 ± 3.2 b | 35.4 ± 18.9 d | 1.94 ± 1.09 b | 25.8 ± 8.0 a | 0.88 ± 0.10 a | 53.9 ± 39.2 f | |
| Gleyic Chernozem | Control | 73.4 ± 5.7 d | 11.8 ± 8.1 b | 331 ± 30 a | 107 ± 15 b | 11.8 ± 2.3 d | 34.4 ± 12.8 c | 4.60 ± 1.73 g | 28.0 ± 8.2 bc | 2.86 ± 2.98 h | 39.2 ± 22.4 b |
| D1 | 70.0 ± 5.6 c | 13.0 ± 8.0 c | 341 ± 40 b | 107 ± 10 b | 13.3 ± 2.2 g | 38.6 ± 12.0 f | 3.82 ± 3.07 ef | 32.2 ± 6.3 gh | 3.73 ± 2.46 j | 62.6 ± 27.2 g | |
| D2 | 65.3 ± 2.4 a | 11.7 ± 7.7 b | 346 ± 21 b | 111 ± 11 c | 10.2 ± 0.8 a | 34.3 ± 13.0 c | 4.40 ± 1.14 g | 32.8 ± 2.4 hi | 1.39 ± 0.10 c | 37.3 ± 16.0 a | |
| D3 | 67.9 ± 2.2 b | 11.1 ± 6.6 a | 386 ± 61 e | 111 ± 17 c | 11.3 ± 2.0 c | 31.4 ± 6.4 a | 2.98 ± 2.93 c | 36.5 ± 8.9 k | 2.19 ± 1.51 f | 51.8 ± 7.2 e | |
| D4 | 80.7 ± 10.1 e | 15.1 ± 8.8 e | 405 ± 78 f | 127 ± 15 g | 13.6 ± 2.8 h | 43.1 ± 15.1 h | 3.15 ± 1.18 c | 34.0 ± 6.4 j | 1.88 ± 0.86 e | 66.1 ± 33.5 i | |
| D5 | 74.1 ± 8.9 d | 14.0 ± 8.7 d | 366 ± 17 cd | 116 ± 9 de | 13.1 ± 2.2 fg | 41.2 ± 13.1 g | 3.17 ± 1.38 c | 33.1 ± 8.2 hij | 1.87 ± 1.53 e | 44.0 ± 24.7 d | |
| D6 | 73.0 ± 8.0 d | 13.0 ± 7.9 c | 328 ± 14 a | 113 ± 13 cd | 12.2 ± 0.8 e | 39.0 ± 9.0 f | 1.21 ± 0.91 a | 33.8 ± 11.3 ij | 3.29 ± 3.45 i | 42.3 ± 25.9 c | |
| Mean for factors | |||||||||||
| Type of Soil (S) | Haplic Luvisol | 86.9 ± 11.0 b | 15.0 ± 11.7 b | 366 ± 49 b | 116 ± 21 b | 12.3 ± 4.0 a | 40.4 ± 20.3 b | 4.02 ± 2.56 b | 29.3 ± 8.7 a | 1.86 ± 1.34 a | 62.7 ± 40.1 b |
| Gleyic Chernozem | 72.1 ± 7.9 a | 12.8 ± 7.7 a | 358 ± 49 a | 113 ± 14 a | 12.2 ± 2.2 a | 37.4 ± 12.0 a | 3.33 ± 2.13 a | 32.9 ± 7.8 b | 2.46 ± 2.19 b | 49.1 ± 25.0 a | |
| Fertilisation (F) | Control | 77.7 ± 8.1 ab | 11.5 ± 7.6 a | 346 ± 49 b | 109 ± 12 b | 11.4 ± 2.6 a | 33.3 ± 13.1 a | 4.93 ± 3.40 e | 27.5 ± 8.5 a | 2.12 ± 2.20 c | 44.9 ± 25.3 a |
| D1 | 78.0 ± 10.0 b | 13.6 ± 9.0 b | 353 ± 44 c | 112 ± 11 c | 11.8 ± 2.2 c | 37.5 ± 12.5 b | 3.84 ± 2.36 c | 31.1 ± 5.6 c | 2.46 ± 2.16 d | 63.4 ± 31.4 f | |
| D2 | 76.5 ± 13.8 a | 13.7 ± 10.6 b | 358 ± 38 c | 116 ± 17 d | 11.7 ± 3.5 bc | 38.5 ± 18.6 c | 3.94 ± 1.77 c | 30.9 ± 6.4 c | 1.90 ± 1.15 b | 49.9 ± 28.3 c | |
| D3 | 79.8 ± 14.8 c | 13.9 ± 11.0 c | 385 ± 51 d | 104 ± 23 a | 12.3 ± 3.7 d | 38.3 ± 19.0 c | 3.94 ± 2.44 c | 33.8 ± 9.4 f | 2.69 ± 1.62 e | 61.5 ± 40.4 e | |
| D4 | 86.6 ± 12.7 d | 15.6 ± 10.0 d | 402 ± 68 e | 130 ± 18 e | 13.9 ± 3.2 f | 45.3 ± 17.6 e | 4.10 ± 2.36 d | 32.7 ± 9.1 e | 2.12 ± 1.42 c | 66.9 ± 40.4 g | |
| D5 | 80.1 ± 13.4 c | 15.5 ± 12.0 d | 355 ± 20 c | 116 ± 17 d | 13.0 ± 4.0 e | 42.3 ± 19.7 d | 3.39 ± 1.32 b | 31.8 ± 8.7 d | 1.76 ± 1.20 a | 56.4 ± 35.9 d | |
| D6 | 77.6 ± 9.3 b | 13.5 ± 10.1 b | 333 ± 23 a | 113 ± 16 c | 11.5 ± 2.3 ab | 37.2 ± 14.5 b | 1.57 ± 1.05 a | 29.8 ± 10.4 b | 2.09 ± 2.67 c | 48.1 ± 32.8 b | |
| Year (Y) | 2019 | 90.0 ± 13.0 c | 27.2 ± 5.4 b | 316 ± 18 a | 128 ± 22 c | 15.7 ± 3.0 b | 59.8 ± 11.6 c | 3.64 ± 1.26 b | 21.6 ± 5.7 a | 0.97 ± 0.28 a | 95.1 ± 26.7 c |
| 2020 | 73.4 ± 6.6 a | 7.2 ± 0.8 a | 380 ± 41 b | 105 ± 11 a | 10.5 ± 0.9 a | 28.6 ± 2.6 b | 4.29 ± 3.39 c | 36.5 ± 4.6 c | 3.79 ± 2.14 c | 38.8 ± 18.0 b | |
| 2021 | 75.0 ± 7.9 b | 7.3 ± 1.5 a | 389 ± 44 c | 110 ± 8 b | 10.4 ± 1.6 a | 28.3 ± 5.6 a | 3.09 ± 1.83 a | 35.3 ± 4.9 b | 1.73 ± 1.12 b | 33.8 ± 10.6 a | |
| Two-way ANOVA (F/p value) | |||||||||||
| S | 5588/0.000 | 3960/0.000 | 57.6/0.000 | 106/0.000 | 2.6/0.111 | 1082/0.000 | 723/0.000 | 828/0.000 | 1043/0.000 | 8527/0.000 | |
| F | 168/0.000 | 891/0.000 | 267.8/0.000 | 468/0.000 | 525/0.000 | 1036/0.000 | 944/0.000 | 150/0.000 | 170/0.000 | 1869/0.000 | |
| Y | 2824/0.000 | 147,330/0.000 | 1773.0/0.000 | 2375/0.000 | 13,841/0.000 | 53,609/0.000 | 731/0.000 | 5784/0.000 | 8309/0.000 | 70,872/0.000 | |
| S*F | 140/0.000 | 539/0.000 | 42.2/0.000 | 121/0.000 | 656/0.000 | 714/0.000 | 225/0.000 | 51/0.000 | 998/0.000 | 641/0.000 | |
| S*Y | 249/0.000 | 6235/0.000 | 87.9/0.000 | 317/0.000 | 2009/0.000 | 4197/0.000 | 2966/0.000 | 279/0.000 | 818/0.000 | 10,271/0.000 | |
| F*Y | 58/0.000 | 342/0.000 | 119.3/0.000 | 222/0.000 | 251/0.000 | 281/0.000 | 995/0.000 | 166/0.000 | 217/0.000 | 1231/0.000 | |
| S*F*Y | 34/0.000 | 430/0.000 | 58.6/0.000 | 329/0.000 | 297/0.000 | 343/0.000 | 606/0.000 | 140/0.000 | 841/0.000 | 817/0.000 | |
| Type of Soil | Fertilisation | Threonine (THR) | Valine (VAL) | Methionine (MET) | Isoleucine (ILE) | Leucine (LEU) | Phenylalanine (PHE) | Histidine (HIS) | Tryptophan (TRYP) | Lysine (LYS) | Arginine (ARG) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg·100 g−1 flour | |||||||||||
| Haplic Luvisol | Control | 8.0 ± 4.1 a | 14.6 ± 4.3 b | 15.5 ± 9.9 ab | 11.2 ± 5.0 b | 34.4 ± 7.8 a | 25.8 ± 3.7 a | 12.7 ± 2.0 bc | 51.0 ± 29.0 cd | 14.8 ± 3.5 b | 34.6 ± 9.8 a |
| D1 | 10.6 ± 6.6 c | 15.9 ± 0.8 cd | 15.4 ± 5.9 ab | 11.2 ± 2.2 b | 41.8 ± 4.5 c | 31.6 ± 4.8 fg | 12.5 ± 2.7 b | 55.0 ± 27.0 fg | 17.5 ± 3.1 c | 39.8 ± 12.1 c | |
| D2 | 11.9 ± 8.3 e | 17.2 ± 4.4 f | 17.0 ± 3.3 c | 12.7 ± 1.7 d | 41.7 ± 6.2 c | 30.0 ± 4.1 de | 13.7 ± 1.7 d | 47.2 ± 25.1 b | 17.3 ± 4.7 d | 38.4 ± 19.7 b | |
| D3 | 12.2 ± 8.4 f | 17.2 ± 3.2 f | 21.6 ± 2.9 g | 14.1 ± 2.6 f | 44.4 ± 6.2 d | 30.9 ± 2.6 ef | 16.2 ± 1.5 h | 50.5 ± 26.8 cd | 19.3 ± 5.6 f | 52.6 ± 18.4 i | |
| D4 | 13.0 ± 7.8 g | 20.3 ± 2.3 j | 20.4 ± 4.0 f | 14.8 ± 3.5 g | 48.4 ± 11.3 e | 35.6 ± 7.5 j | 15.5 ± 1.2 g | 45.1 ± 21.2 a | 22.6 ± 4.2 j | 55.8 ± 16.9 j | |
| D5 | 12.4 ± 9.6 f | 16.4 ± 3.5 e | 19.2 ± 3.6 e | 13.5 ± 3.6 e | 43.7 ± 8.0 d | 29.9 ± 2.3 d | 12.5 ± 0.7 b | 51.2 ± 27.9 cd | 20.1 ± 3.9 g | 44.9 ± 14.7 f | |
| D6 | 10.0 ± 7.7 b | 13.1 ± 2.3 a | 15.8 ± 3.1 b | 10.5 ± 2.6 a | 34.4 ± 8.5 a | 25.1 ± 5.2 a | 11.7 ± 1.5 a | 51.0 ± 28.0 cd | 15.9 ± 4.1 b | 42.1 ± 16.0 e | |
| Gleyic Chernozem | Control | 9.9 ± 5.9 b | 15.7 ± 1.7 c | 17.6 ± 5.0 cd | 12.1 ± 2.2 c | 39.8 ± 5.4 b | 28.8 ± 5.1 c | 13.8 ± 2.1 d | 51.6 ± 19.3 de | 16.7 ± 3.5 c | 35.3 ± 10.6 a |
| D1 | 10.5 ± 4.9 c | 18.9 ± 1.6 hi | 19.8 ± 5.2 ef | 14.2 ± 2.6 f | 47.9 ± 6.7 e | 34.7 ± 3.5 i | 14.4 ± 3.7 e | 55.8 ± 23.7 g | 18.3 ± 3.6 e | 40.1 ± 10.5 cd | |
| D2 | 9.9 ± 5.7 b | 16.2 ± 4.3 de | 17.3 ± 2.6 cd | 13.3 ± 1.8 e | 41.6 ± 6.8 c | 27.4 ± 4.5 b | 14.7 ± 2.5 ef | 56.1 ± 23.1 g | 16.8 ± 6.9 c | 37.6 ± 11.0 b | |
| D3 | 10.7 ± 6.3 c | 17.8 ± 2.5 g | 15.2 ± 4.8 ab | 15.6 ± 3.3 h | 44.8 ± 5.1 d | 31.8 ± 7.3 g | 16.2 ± 3.4 h | 50.6 ± 23.0 cd | 20.0 ± 6.5 g | 42.0 ± 9.0 e | |
| D4 | 11.3 ± 4.6 d | 19.3 ± 2.5 i | 15.1 ± 2.5 a | 12.5 ± 1.9 d | 49.0 ± 10.4 e | 36.6 ± 8.0 k | 16.7 ± 1.6 i | 49.7 ± 22.5 c | 22.3 ± 4.7 ij | 50.7 ± 7.0 h | |
| D5 | 11.2 ± 5.6 d | 18.9 ± 1.8 h | 15.7 ± 4.8 ab | 13.3 ± 1.3 e | 50.9 ± 9.2 f | 37.8 ± 6.9 l | 15.0 ± 0.4 f | 56.2 ± 22.8 g | 21.5 ± 5.4 h | 46.6 ± 12.9 g | |
| D6 | 10.7 ± 5.4 c | 18.6 ± 1.5 h | 17.9 ± 6.3 d | 13.4 ± 3.4 e | 56.0 ± 14.9 g | 33.3 ± 7.7 h | 13.1 ± 1.8 c | 53.4 ± 23.8 ef | 21.8 ± 3.4 hi | 41.1 ± 7.3 de | |
| Mean for factors | |||||||||||
| Type of Soil (S) | Haplic Luvisol | 11.2 ± 7.5 b | 16.4 ± 3.7 a | 17.8 ± 5.5 b | 12.6 ± 3.4 a | 41.2 ± 8.8 a | 29.9 ± 5.5 a | 13.6 ± 2.3 a | 50.1 ± 25.4 a | 18.2 ± 4.7 a | 44.0 ± 16.6 b |
| Gleyic Chernozem | 10.6 ± 5.3 a | 17.9 ± 2.7 b | 16.9 ± 4.7 a | 13.5 ± 2.6 b | 47.1 ± 10.0 b | 32.9 ± 7.0 b | 14.8 ± 2.6 b | 53.3 ± 21.7 b | 19.6 ± 5.3 b | 41.9 ± 10.7 a | |
| Fertilisation (F) | Control | 8.9 ± 5.0 a | 15.1 ± 3.2 a | 16.5 ± 7.7 a | 11.7 ± 3.8 a | 37.1 ± 7.1 a | 27.3 ± 4.6 a | 13.2 ± 2.0 b | 51.3 ± 23.9 bc | 15.7 ± 3.5 b | 35.0 ± 9.9 a |
| D1 | 10.5 ± 5.6 c | 17.4 ± 2.0 d | 17.6 ± 5.8 e | 12.7 ± 2.8 c | 44.8 ± 6.4 c | 33.2 ± 4.4 d | 13.5 ± 3.3 b | 55.4 ± 24.7 e | 17.9 ± 3.3 c | 40.0 ± 11.0 c | |
| D2 | 10.9 ± 7.0 d | 16.7 ± 4.3 c | 17.1 ± 2.9 cd | 13.0 ± 1.7 d | 41.6 ± 6.3 b | 28.7 ± 4.4 b | 14.2 ± 2.1 d | 51.6 ± 23.8 bc | 17.0 ± 5.7 b | 38.0 ± 15.5 b | |
| D3 | 11.5 ± 7.3 e | 17.5 ± 2.8 d | 18.4 ± 5.1 f | 14.8 ± 3.0 g | 44.6 ± 5.5 c | 31.3 ± 5.3 c | 16.2 ± 2.5 e | 50.5 ± 24.2 b | 19.7 ± 5.9 e | 47.3 ± 15.0 f | |
| D4 | 12.2 ± 6.3 g | 19.8 ± 2.3 e | 17.7 ± 4.2 e | 13.7 ± 3.0 f | 48.7 ± 10.6 e | 36.1 ± 7.5 f | 16.1 ± 1.5 e | 47.4 ± 20.4 a | 22.4 ± 4.3 g | 53.3 ± 12.8 g | |
| D5 | 11.8 ± 7.7 f | 17.7 ± 3.0 d | 17.5 ± 4.5 de | 13.4 ± 2.6 e | 47.3 ± 9.2 d | 33.8 ± 6.4 e | 13.8 ± 1.4 c | 53.7 ± 24.9 d | 20.8 ± 4.6 f | 45.7 ± 13.4 e | |
| D6 | 10.3 ± 6.5 b | 15.8 ± 3.4 b | 16.8 ± 4.9 ab | 12.0 ± 3.3 b | 45.2 ± 16.2 c | 29.2 ± 7.6 b | 12.4 ± 1.7 a | 52.2 ± 25.2 c | 18.8 ± 4.8 d | 41.6 ± 12.1 d | |
| Year (Y) | 2019 | 19.5 ± 3.1 b | 18.5 ± 2.4 c | 16.1 ± 3.2 b | 11.2 ± 2.8 a | 37.4 ± 5.6 a | 26.5 ± 3.6 a | 15.0 ± 2.0 c | 20.3 ± 5.0 a | 23.5 ± 2.9 c | 59.4 ± 9.3 c |
| 2020 | 6.6 ± 0.8 a | 17.7 ± 2.4 b | 20.7 ± 4.7 c | 15.4 ± 1.9 c | 49.7 ± 5.9 c | 34.2 ± 2.9 c | 14.2 ± 2.7 b | 66.7 ± 7.4 b | 16.5 ± 2.5 a | 33.7 ± 6.9 a | |
| 2021 | 6.6 ± 1.4 a | 15.2 ± 4.0 a | 15.3 ± 5.5 a | 12.4 ± 2.7 b | 45.4 ± 12.2 b | 33.5 ± 8.4 b | 13.4 ± 2.7 a | 68.2 ± 9.4 c | 16.8 ± 5.6 b | 35.8 ± 6.3 b | |
| Two-way ANOVA (F/p value) | |||||||||||
| S | 533/0.000 | 1144/0.000 | 164/0.000 | 591/0.000 | 2061/0.000 | 1013/0.000 | 1129/0.000 | 258/0.000 | 744/0.000 | 360/0.000 | |
| F | 1083/0.000 | 613/0.000 | 46/0.000 | 428/0.000 | 498/0.000 | 627/0.000 | 845/0.000 | 92/0.000 | 1065/0.000 | 1806/0.000 | |
| Y | 120,562/0.000 | 1859/0.000 | 2263/0.000 | 3978/0.000 | 3113/0.000 | 2600/0.000 | 595/0.000 | 25,151/0.000 | 7423/0.000 | 22,049/0.000 | |
| S*F | 473/0.000 | 380/0.000 | 500/0.000 | 317/0.000 | 481/0.000 | 236/0.000 | 61/0.000 | 36/0.000 | 243/0.000 | 217/0.000 | |
| S*Y | 3350/0.000 | 1669/0.000 | 1443/0.000 | 1352/0.000 | 553/0.000 | 86/0.000 | 651/0.000 | 237/0.000 | 1022/0.000 | 1372/0.000 | |
| F*Y | 397/0.000 | 401/0.000 | 437/0.000 | 355/0.000 | 396/0.000 | 334/0.000 | 184/0.000 | 112/0.000 | 452/0.000 | 106/0.000 | |
| S*F*Y | 404/0.000 | 148/0.000 | 403/0.000 | 316/0.000 | 224/0.000 | 204/0.000 | 528/0.000 | 222/0.000 | 437/0.000 | 166/0.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czernicka, M.; Puchalski, C.; Pawlak, R.; Szostek, M.; Szpunar-Krok, E. Analysis of the Free Amino Acid Profile of Barley Grain from Organic Fertilisation with Ash from Biomass Combustion. Molecules 2024, 29, 95. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules29010095
Czernicka M, Puchalski C, Pawlak R, Szostek M, Szpunar-Krok E. Analysis of the Free Amino Acid Profile of Barley Grain from Organic Fertilisation with Ash from Biomass Combustion. Molecules. 2024; 29(1):95. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules29010095
Chicago/Turabian StyleCzernicka, Maria, Czesław Puchalski, Renata Pawlak, Małgorzata Szostek, and Ewa Szpunar-Krok. 2024. "Analysis of the Free Amino Acid Profile of Barley Grain from Organic Fertilisation with Ash from Biomass Combustion" Molecules 29, no. 1: 95. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules29010095