Surface-Active Ionic Liquids and Surface-Active Quaternary Ammonium Salts from Synthesis, Characterization to Antimicrobial Properties
Abstract
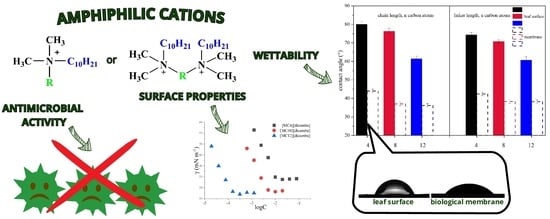
:1. Introduction
2. Results and Discussion
2.1. Thermal Analysis
2.2. Self-Aggregation in Water Solution
2.3. Wettability
2.4. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of Alkyldecyldimethylammonium Bromide
3.2.2. Synthesis of Alkane-1,ω-bis(Decyldimethylammonium) Dibromide
3.2.3. Metathesis Reaction
3.3. Thermal Analysis
3.4. Self-Aggregation in Water Solution
3.5. Wettability
3.6. Statistical Analysis
3.7. Preparation of Membrane
3.8. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, M.K.; Moshikur, R.M.; Goto, M. Surface-Active Ionic Liquids for Medical and Pharmaceutical Applications. In Application of Ionic Liquids in Drug Delivery; Springer: Singapore, 2021; pp. 165–186. [Google Scholar]
- Pillai, P.; Mandal, A. Synthesis and Characterization of Surface-Active Ionic Liquids for Their Potential Application in Enhanced Oil Recovery. J. Mol. Liq. 2022, 345, 117900. [Google Scholar] [CrossRef]
- Buettner, C.S.; Cognigni, A.; Schröder, C.; Bica-Schröder, K. Surface-Active Ionic Liquids: A Review. J. Mol. Liq. 2022, 347, 118160. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Wojcieszak, M.; Lewandowska, A.; Marcinkowska, A.; Pałkowski, Ł.; Karolak, M.; Skrzypczak, A.; Syguda, A.; Materna, K. Evaluation of Antimicrobial Properties of Monocationic and Dicationic Surface-Active Ionic Liquids. J. Mol. Liq. 2023, 374, 121300. [Google Scholar] [CrossRef]
- Kharazi, M.; Saien, J. Mechanism Responsible Altering in Interfacial Tension and Emulsification of the Crude Oil-Water System with Nano Gemini Surface Active Ionic Liquids, Salts and PH. J. Pet. Sci. Eng. 2022, 219, 111090. [Google Scholar] [CrossRef]
- Kharazi, M.; Saien, J.; Torabi, M.; Zolfigol, M.A. Molecular Design and Applications of a Nanostructure Green Tripodal Surface Active Ionic Liquid in Enhanced Oil Recovery: Interfacial Tension Reduction, Wettability Alteration, and Emulsification. Pet. Sci. 2023, 20, 3530–3539. [Google Scholar] [CrossRef]
- Wȩgrzyńska, J.; Chlebicki, J. Preparation, Surface-Active and Antielectrostatic Properties of Multiple Quaternary Ammonium Salts. J. Surfactants Deterg. 2006, 9, 221–226. [Google Scholar] [CrossRef]
- Chlebicki, J.; Węgrzyńska, J.; Maliszewska, I.; Oświęcimska, M. Preparation, Surface-Active Properties, and Antimicrobial Activities of Bis-Quaternary Ammonium Salts from Amines and Epichlorohydrin. J. Surfactants Deterg. 2005, 8, 227–232. [Google Scholar] [CrossRef]
- Frizzo, C.P.; Gindri, I.M.; Bender, C.R.; Tier, A.Z.; Villetti, M.A.; Rodrigues, D.C.; Machado, G.; Martins, M.A.P. Effect on Aggregation Behavior of Long-Chain Spacers of Dicationic Imidazolium-Based Ionic Liquids in Aqueous Solution. Colloids Surf. A Physicochem. 2015, 468, 285–294. [Google Scholar] [CrossRef]
- Shirota, H.; Mandai, T.; Fukazawa, H.; Kato, T. Comparison between Dicationic and Monocationic Ionic Liquids: Liquid Density, Thermal Properties, Surface Tension, and Shear Viscosity. J. Chem. Eng. Data 2011, 56, 2453–2459. [Google Scholar] [CrossRef]
- Baltazar, Q.Q.; Chandawalla, J.; Sawyer, K.; Anderson, J.L. Interfacial and Micellar Properties of Imidazolium-Based Monocationic and Dicationic Ionic Liquids. Colloids Surf. A Physicochem. 2007, 302, 150–156. [Google Scholar] [CrossRef]
- Ohno, H. Functional Design of Ionic Liquids. Bull. Chem. Soc. Jpn. 2006, 79, 1665–1680. [Google Scholar] [CrossRef]
- Saien, J.; Kharazi, M.; Pino, V.; Pacheco-Fernández, I. Trends Offered by Ionic Liquid-Based Surfactants: Applications in Stabilization, Separation Processes, and within the Petroleum Industry. Sep. Purif. Rev. 2023, 52, 164–192. [Google Scholar] [CrossRef]
- Pérez, L.; Pinazo, A.; Vinardell, P.; Clapés, P.; Angelet, M.; Infante, M.R. Synthesis and Biological Properties of Dicationic Arginine-Diglycerides. New J. Chem. 2002, 26, 1221–1227. [Google Scholar] [CrossRef]
- Wojcieszak, M.; Kaczmarek, D.K.; Krzyźlak, K.; Siarkiewicz, A.; Klejdysz, T.; Materna, K. Surface Properties of Dicationic Ionic Liquids and Correlation with Biological Activity. Tenside Surfactants Deterg. 2022, 59, 294–304. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Razuvayeva, Y.S.; Ziganshina, A.Y.; Mukhitova, R.K.; Sapunova, A.S.; Voloshina, A.D.; Zakharova, L.Y. Self-Assembling and Biological Properties of Single-Chain Dicationic Pyridinium-Based Surfactants. Colloids Surf. B. 2019, 175, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kharazi, M.; Saien, J.; Asadabadi, S. Review on Amphiphilic Ionic Liquids as New Surfactants: From Fundamentals to Applications. Top. Curr. Chem. 2022, 380, 5. [Google Scholar] [CrossRef]
- Pałkowski, Ł.; Karolak, M.; Skrzypczak, A.; Wojcieszak, M.; Walkiewicz, F.; Podemski, J.; Jaroch, K.; Bojko, B.; Materna, K.; Krysiński, J. Antimicrobial and Cytotoxic Activity of Novel Imidazolium-Based Ionic Liquids. Molecules 2022, 27, 1974. [Google Scholar] [CrossRef]
- Cao, H.; Hu, Y.; Xu, W.; Wang, Y.; Guo, X. Recent Progress in the Assembly Behavior of Imidazolium-Based Ionic Liquid Surfactants. J. Mol. Liq. 2020, 319, 114354. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Perez, L.; Manresa, A.; Comelles, F. Aggregation Behavior and Antimicrobial Activity of Ester-Functionalized Imidazolium- and Pyridinium-Based Ionic Liquids in Aqueous Solution. Langmuir 2013, 29, 2536–2545. [Google Scholar] [CrossRef]
- Shaheen, A.; Arif, R.; Mir, A.W.; Rehman, S. Synthesis, Physicochemical Characteristics and Antimicrobial Studies of Ethyl-Substituted Imidazolium-Based Surface Active Ionic Liquids (SAILs). Colloids Interface Sci. Commun. 2019, 33, 100204. [Google Scholar] [CrossRef]
- Demberelnyamba, D.; Kim, K.S.; Choi, S.; Park, S.Y.; Lee, H.; Kim, C.J.; Yoo, I.D. Synthesis and Antimicrobial Properties of Imidazolium and Pyrrolidinonium Salts. Bioorg. Med. Chem. 2004, 12, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, M.; Krupa, B.; Syguda, A.; Walkiewicz, F.; Wilkowska, M.; Kozak, M.; Materna, K. Surface Activity and Phytotoxicity of Morpholinium Herbicidal Ionic Liquids. J. Mol. Liq. 2022, 362, 119750. [Google Scholar] [CrossRef]
- Bureš, F. Quaternary Ammonium Compounds: Simple in Structure, Complex in Application. Top. Curr. Chem. 2019, 377, 14. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, M.; Syguda, A.; Lewandowska, A.; Marcinkowska, A.; Siwińska-Ciesielczyk, K.; Wilkowska, M.; Kozak, M.; Materna, K. Synthesis and Surface Properties of Piperidinium-Based Herbicidal Ionic Liquids as a Potential Tool for Weed Control. J. Agric. Food Chem. 2023, 71, 4550–4560. [Google Scholar] [CrossRef]
- Kawai, R.; Yada, S.; Yoshimura, T. Characterization and Solution Properties of Quaternary-Ammonium-Salt-Type Amphiphilic Gemini Ionic Liquids. ACS Omega 2019, 4, 14242–14250. [Google Scholar] [CrossRef]
- Coker, T.G.; Wunderlich, B.; Janz, G.J. Melting Mechanisms of Ionic Salts. Tetra-n-Amyl Ammonium Thiocyanate. Trans. Faraday Soc. 1969, 65, 3361–3368. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 1. Variation of Anionic Species. J. Phys. Chem. B 2004, 108, 16593–16600. [Google Scholar] [CrossRef]
- Yuli, I.; Tzafrir, I.; Salama, P. Compatibility Investigation of Cationic Surfactants with Anionic Species. Cosmetics 2023, 10, 45. [Google Scholar] [CrossRef]
- Khatua, D.; Gupta, A.; Dey, J. Characterization of Micelle Formation of Dodecyldimethyl-N-2-Phenoxyethylammonium Bromide in Aqueous Solution. J. Colloid. Interface Sci. 2006, 298, 451–456. [Google Scholar] [CrossRef]
- Yoshimura, T.; Yoshida, H.; Ohno, A.; Esumi, K. Physicochemical Properties of Quaternary Ammonium Bromide-Type Trimeric Surfactants. J. Colloid. Interface Sci. 2003, 267, 167–172. [Google Scholar] [CrossRef]
- Kapitanov, I.V.; Jordan, A.; Karpichev, Y.; Spulak, M.; Perez, L.; Kellett, A.; Kümmerer, K.; Gathergood, N. Synthesis, Self-Assembly, Bacterial and Fungal Toxicity, and Preliminary Biodegradation Studies of a Series of l-Phenylalanine-Derived Surface-Active Ionic Liquids. Green. Chem. 2019, 21, 1777–1794. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; Dong, J.; Zhang, G.; Wang, C. A Novel Family of Green Ionic Liquids with Surface Activities. Sci. China Chem. 2007, 50, 238–242. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, L.; Wang, T.; Chen, P.; Bi, Y.; Yu, L. Aggregation Behavior of Dodecylsulfonate-Based Surface Active Ionic Liquids in Water. J. Mol. Liq. 2015, 212, 23–29. [Google Scholar] [CrossRef]
- Bera, A.; Kumar, T.; Ojha, K.; Mandal, A. Adsorption of Surfactants on Sand Surface in Enhanced Oil Recovery: Isotherms, Kinetics and Thermodynamic Studies. Appl. Surf. Sci. 2013, 284, 87–99. [Google Scholar] [CrossRef]
- Dong, B.; Li, N.; Zheng, L.; Yu, L.; Inoue, T. Surface Adsorption and Micelle Formation of Surface Active Ionic Liquids in Aqueous Solution. Langmuir 2007, 23, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Zdziennicka, A.; Krawczyk, J.; Szymczyk, K.; Jańczuk, B. Macroscopic and Microscopic Properties of Some Surfactants and Biosurfactants. Int. J. Mol. Sci. 2018, 19, 1934. [Google Scholar] [CrossRef] [PubMed]
- Jańczuk, B.; Sierra, J.A.M.; González-Martín, M.L.; Bruque, J.M.; Wójcik, W. Properties of decylammonium chloride and cesium perfluorooctanoate at interfaces and standard free energy of their adsorption. J. Colloid. Interface Sci. 1997, 192, 408–414. [Google Scholar] [CrossRef]
- Szaniawska, M.; Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Thermodynamic Parameters of Berberine with Kolliphor Mixtures Adsorption and Micellization. Molecules 2023, 28, 3115. [Google Scholar] [CrossRef]
- Moselhy, M.A.; Zaki, E.G.; El-Maksoud, S.A.E.H.A.; Migahed, M.A. Surface Activity and Electrochemical Behavior of Some Thiazine Cationic Surfactants and Their Efficiency as Corrosion Inhibitors for Carbon Steel in a Sour Environment. ACS Omega 2021, 30, 19559–19568. [Google Scholar] [CrossRef]
- Danov, K.D.; Kralchevsky, P.A. The standard free energy of surfactant adsorption at air/water and oil/water interfaces: Theoretical vs. empirical approaches. Colloid. J. 2012, 74, 172–185. [Google Scholar] [CrossRef]
- Singh, G.; Singh, G.; Kang, T.S. Micellization Behavior of Surface Active Ionic Liquids Having Aromatic Counterions in Aqueous Media. J. Phys. Chem. B 2016, 120, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Chabba, S.; Kumar, S.; Aswal, V.K.; Kang, T.S.; Mahajan, R.K. Interfacial and Aggregation Behavior of Aqueous Mixtures of Imidazolium Based Surface Active Ionic Liquids and Anionic Surfactant Sodium Dodecylbenzenesulfonate. Colloids Surf. A Physicochem. Eng. 2015, 472, 9–20. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Yu, L.; Jiao, J.; Wang, R.; Sun, L. Surface Adsorption and Micelle Formation of Imidazolium-Based Zwitterionic Surface Active Ionic Liquids in Aqueous Solution. J. Colloid. Interface Sci. 2013, 391, 103–110. [Google Scholar] [CrossRef]
- Asadov, Z.H.; Huseynova, S.M.; Ahmadova, G.A.; Rahimov, R.A.; Sharbatov, S.U.; Zubkov, F.I.; Jafarova, R.A. Synthesis, Colloidal-Chemical and Petroleum Collecting Properties of New Counterion Coupled Gemini Surfactants Based on Hexadecylbis(2-Hydroxypropyl)Amine and Dicarboxylic Acids. J. Dispers. Sci. Technol. 2020, 41, 2063–2071. [Google Scholar] [CrossRef]
- Arabloo, M.; Ghazanfari, M.H.; Rashtchian, D. Wettability Modification, Interfacial Tension and Adsorption Characteristics of a New Surfactant: Implications for Enhanced Oil Recovery. Fuel 2016, 185, 199–210. [Google Scholar] [CrossRef]
- Song, J.W.; Ma, M.C.; Fan, L.W. Understanding the Temperature Dependence of Contact Angles of Water on a Smooth Hydrophobic Surface under Pressurized Conditions: An Experimental Study. Langmuir 2020, 36, 9586–9595. [Google Scholar] [CrossRef]
- Song, J.W.; Fan, L.W. Temperature Dependence of the Contact Angle of Water: A Review of Research Progress, Theoretical Understanding, and Implications for Boiling Heat Transfer. Adv. Colloid. Interface Sci. 2021, 288, 102339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, T.; Zeng, J.; Yin, X.; Lan, Y.; Wen, S. Effects of Temperature and Humidity on the Contact Angle of Pesticide Droplets on Rice Leaf Surfaces. J. Pestic. Sci. 2022, 47, 59–68. [Google Scholar] [CrossRef]
- Zhang, C.H.; Zhao, X.; Lei, J.M.; Ma, Y.; Du, F.P. Wettability of Triton X-100 on Wheat (Triticum Aestivum) Leaf Surfaces with Respect to Developmental Changes. Acta Phys.-Chim. Sin. 2017, 33, 1846–1854. [Google Scholar]
- Rana, S.; Kamboj, J.K.; Gandhi, V. Living Life the Natural Way—Wheatgrass and Health. Funct. Foods Health Dis. 2011, 1, 444–456. [Google Scholar] [CrossRef]
- Skiba, E.; Wolf, W.M. Commercial Phenoxyacetic Herbicides Control Heavy Metal Uptake by Wheat in a Divergent Way than Pure Active Substances Alone. Environ. Sci. Eur. 2017, 29, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Costa-Gutierrez, S.B.; Adler, C.; Espinosa-Urgel, M.; de Cristóbal, R.E. Pseudomonas Putida and Its Close Relatives: Mixing and Mastering the Perfect Tune for Plants. Appl. Microbiol. Biotechnol. 2022, 106, 3351–3367. [Google Scholar] [CrossRef] [PubMed]
- Cornellas, A.; Perez, L.; Comelles, F.; Ribosa, I.; Manresa, A.; Garcia, T.T. Self-Aggregation and Antimicrobial Activity of Imidazolium and Pyridinium Based Ionic Liquids in Aqueous Solution. J. Colloid. Interface Sci. 2011, 355, 164–171. [Google Scholar] [CrossRef] [PubMed]
- CLSI Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Garcia, M.T.; Ribosa, I.; González, J.J.; Comelles, F. Surface Activity, Self-Aggregation and Antimicrobial Activity of Catanionic Mixtures of Surface Active Imidazolium- or Pyridinium-Based Ionic Liquids and Sodium Bis(2-Ethylhexyl) Sulfosuccionate. J. Mol. Liq. 2020, 303, 112637. [Google Scholar] [CrossRef]








| Abbreviation | Tcc1 (°C) | Tc1 (°C) | Tc2 (°C) | Ts-s1 (°C) | Ts-s2 (°C) | Tm (°C) | Tg (°C) | T5% (°C) | T50% (°C) |
|---|---|---|---|---|---|---|---|---|---|
| [MC4][dicamba] | 61.00 | 66.17 | - | - | - | 83.83 | −39.52 | 170.5 | 212.4 |
| [MC8][dicamba] | - | 38.83 | - | - | - | 67.33 | −59.20 | 165.5 | 215.5 |
| [MC12][dicamba] | - | 10.33 | 65.00 | 28.83 | 46.17 | 68.83 | −51.87 | 168.0 | 224.7 |
| [DC4][dicamba]2 | 53.33 | 75.33 | - | - | - | 86.83 | −2.53 | 175.4 | 216.9 |
| [DC8][dicamba]2 | - | 73.33 | - | - | - | 134.30 | −10.04 | 177.0 | 243.1 |
| [DC12][dicamba]2 | - | 49.33 | - | - | - | 121.50 | - | 186.6 | 263.0 |
| Abbreviation | CMC (mmol L−1) | pC20 | γCMC (mN m−1) | ΠCMC (mN m−1) | Γmax × 106 (mol m−2) | Amin × 1019 (m2) | ΔG0ads (kJ mol−1) |
|---|---|---|---|---|---|---|---|
| [MC4][dicamba] | 13.49 | 2.71 | 34.5 | 38.3 | 4.95 | 3.35 | −18.74 |
| [MC8][dicamba] | 4.70 | 3.39 | 28.8 | 44.0 | 3.93 | 4.22 | −24.12 |
| [MC12][dicamba] | 0.13 | 4.86 | 28.0 | 44.8 | 4.52 | 3.67 | −30.87 |
| [DC4][dicamba]2 | 1.76 | 3.54 | 31.8 | 41.0 | 4.81 | 3.46 | −23.88 |
| [DC8][dicamba]2 | 1.48 | 3.66 | 35.9 | 36.9 | 2.98 | 5.57 | −27.46 |
| [DC12][dicamba]2 | 1.05 | 3.99 | 37.0 | 35.8 | 2.67 | 6.23 | −30.00 |
| DDAC | 2.00 a | ||||||
| DomphB | 1.78 b | ||||||
| C10TAB | 67.00 c | ||||||
| C12TAB | 15.00 c |
| MIC (mmol L−1) | |||||||
|---|---|---|---|---|---|---|---|
| Human Pathogenic Microorganisms | Soil Microorganism | ||||||
| G(+) | G(−) | Fungi | G(−) | ||||
| Abbreviation | SAU | EFA | PAE | ECO | KPN | CAL | PPT |
| [MC4][dicamba] | 0.173 | 0.346 | 10.811 | 2.703 | 10.811 | 0.670 | 2.703 |
| [MC8][dicamba] | 0.019 | 0.039 | 1.196 | 0.154 | 0.309 | 0.039 | 0.077 |
| [MC12][dicamba] | 0.002 | 0.004 | 4.350 | 1.079 | 1.079 | 0.004 | 1.079 |
| [DC4][dicamba]2 | 0.006 | 0.003 | 0.715 | 0.092 | 1.442 | 0.006 | 0.023 |
| [DC8][dicamba]2 | 0.011 | 0.011 | 0.336 | 0.011 | 0.087 | 0.011 | 0.087 |
| [DC12][dicamba]2 | 0.003 | 0.003 | 0.082 | 0.005 | 0.041 | 0.003 | 0.041 |
| DDAC | 0.004 | 0.007 | 0.856 | 0.014 | 0.856 | 0.007 | 0.442 |
| BAC | 0.028 | 0.014 | 1.765 | 0.057 | 0.456 | 0.014 | 0.459 |
| Abbreviation | -R | Anion | Yield (%) | Abbreviation | -R- | Anion | Yield (%) |
| [MC4][Br] | -C4H9 | Br | 90 | [DC4][Br]2 | -C4H8- | Br | 89 |
| [MC8][Br] | -C8H17 | 85 | [DC8][Br]2 | -C8H16- | 90 | ||
| [MC12][Br] | -C12H25 | 89 | [DC12][Br]2 | -C12H24- | 91 | ||
| Abbreviation | -R | Anion | Yield (%) | Abbreviation | -R- | Anion | Yield (%) |
| [MC4][dicamba] | -C4H9 | Dicamba | 95 | [DC4][dicamba]2 | -C4H8- | Dicamba | 92 |
| [MC8][dicamba] | -C8H17 | 96 | [DC8][dicamba]2 | -C8H16- | 90 | ||
| [MC12][dicamba] | -C12H25 | 94 | [DC12][dicamba]2 | -C12H24- | 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojcieszak, M.; Kaczmarek, D.K.; Karolak, M.; Pałkowski, Ł.; Lewandowska, A.; Marcinkowska, A.; Dopierała, K.; Materna, K. Surface-Active Ionic Liquids and Surface-Active Quaternary Ammonium Salts from Synthesis, Characterization to Antimicrobial Properties. Molecules 2024, 29, 443. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules29020443
Wojcieszak M, Kaczmarek DK, Karolak M, Pałkowski Ł, Lewandowska A, Marcinkowska A, Dopierała K, Materna K. Surface-Active Ionic Liquids and Surface-Active Quaternary Ammonium Salts from Synthesis, Characterization to Antimicrobial Properties. Molecules. 2024; 29(2):443. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules29020443
Chicago/Turabian StyleWojcieszak, Marta, Damian Krystian Kaczmarek, Maciej Karolak, Łukasz Pałkowski, Aneta Lewandowska, Agnieszka Marcinkowska, Katarzyna Dopierała, and Katarzyna Materna. 2024. "Surface-Active Ionic Liquids and Surface-Active Quaternary Ammonium Salts from Synthesis, Characterization to Antimicrobial Properties" Molecules 29, no. 2: 443. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules29020443