Sodium N-(3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carbonyl)-l-methioninate
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
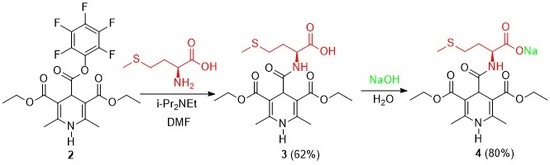
3.1. N-(3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carbonyl)-l-methionine (3)
3.2. Sodium N-(3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carbonyl)-l-methioninate (4)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, V.K.; Singh, S.K. Synthesis, utility and medicinal importance of 1,2- & 1,4-dihydropyridines. RSC Adv. 2017, 7, 2682–2732. [Google Scholar] [CrossRef] [Green Version]
- Godfraind, T. Calcium channel blockers in cardiovascular pharmacotherapy. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Misane, I.; Klusa, V.; Dambrova, M.; Germane, S.; Duburs, G.; Bisenieks, E.; Rimondini, R.; Ögren, S.O. “Atypical” neuromodulatory profile of glutapyrone, a representative of a novel “class” of amino acid-containing dipeptide-mimicking 1,4-dihydropyridine (DHP) compounds: In vitro and in vivo studies. Eur. Neuropsychopharmacol. 1998, 8, 329–347. [Google Scholar] [CrossRef]
- Klusa, V. Atypical 1,4-dihydropyridine derivatives, an approach to neuroprotection and memory enhancement. Pharmacol. Res. 2016, 113, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Ošiņa, K.; Rostoka, E.; Sokolovska, J.; Paramonova, N.; Bisenieks, E.; Duburs, G.; Sjakste, N.; Sjakste, T. 1,4-Dihydropyridine derivatives without Ca2+-antagonist activity up-regulate Psma6 mRNA expression in kidneys of intact and diabetic rats. Cell Biochem. Funct. 2016, 34, 3–6. [Google Scholar] [CrossRef]
- Dzirkale, Z.; Pupure, J.; Rumaks, J.; Svirskis, S.; Vanina, M.; Mezhapuke, R.; Sile, V.; Fernandes, M.A.; Duburs, G.; Klusa, V. Comparative study of taurine and tauropyrone: GABA receptor binding, mitochondrial processes and behaviour. J. Pharm. Pharmacol. 2011, 63, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Borovic, S.; Tirzitis, G.; Tirzite, D.; Cipak, A.; Khoschsorur, G.A.; Waeg, G.; Tatzber, F.; Scukanec-Spoljar, M.; Zarkovic, N. Bioactive 1,4-dihydroisonicotinic acid derivatives prevent oxidative damage of liver cells. Eur. J. Pharmacol. 2006, 537, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, V.; Narbute, K.; Amara, I.; Trovato, A.; Scuto, M.; Pupure, J.; Jansone, B.; Poikans, J.; Bisenieks, E.; Klusa, V.; et al. GABA-containing compound gammapyrone protects against brain impairments in Alzheimer’s disease model male rats and prevents mitochondrial dysfunction in cell culture. J. Neurosci. Res. 2019, 97, 708–726. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Huang, H.; Li, Z.; Liu, X.; Fan, W.; Wang, X.; Sun, X.; Zhu, J.; Zhou, H.; Wei, H. Taurine Promotes the Cartilaginous Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Vitro. Neurochem. Res. 2017, 42, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolev, A.; Franssen, M.C.R.; Poikans, J.; Duburs, G.; de Groot, A. Enantioselective lipase-catalysed kinetic resolution of acyloxymethyl and ethoxycarbonylmethyl esters of 1,4-dihydroisonicotinic acid derivatives. Tetrahedron Asymmetry 2002, 13, 2389–2397. [Google Scholar] [CrossRef]
- Sobolev, A.; Franssen, M.C.R.; Duburs, G.; De Groot, A.E. Chemoenzymatic synthesis of enantiopure 1,4-dihydropyridine derivatives. Biocatal. Biotransformation 2004, 22. [Google Scholar] [CrossRef]
- Ramapanicker, R.; Baig, N.B.R.; De, K.; Chandrasekaran, S. One-pot protection and activation of amino acids using pentafluorophenyl carbonates. J. Pept. Sci. 2009, 15, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. The Reactions of Bioconjugation. In Bioconjugate Techniques; Elsevier: Amsterdam, The Netherlands, 2013; pp. 229–258. [Google Scholar]
- Alandini, N.; Buzzetti, L.; Favi, G.; Schulte, T.; Candish, L.; Collins, K.D.; Melchiorre, P. Amide Synthesis by Nickel/Photoredox-Catalyzed Direct Carbamoylation of (Hetero)Aryl Bromides. Angew. Chem. Int. Ed. 2020, 59, 5248–5253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeitz, J.O.; Kaltenböck, S.; Most, E.; Eder, K. Effects of l-methionine on performance, gut morphology and antioxidant status in gut and liver of piglets in relation to dl-methionine. J. Anim. Physiol. Anim. Nutr. 2019, 103, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wunsch, E.; Raszeja-Wyszomirska, J.; Barbier, O.; Milkiewicz, M.; Krawczyk, M.; Milkiewicz, P. Effect of S-adenosyl-l-methionine on liver biochemistry and quality of life in patients with primary biliary cholangitis treated with ursodeoxycholic acid. A prospective, open label pilot study. J. Gastrointest. Liver Dis. 2018, 27, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, Y.; Chen, W.; Liu, X.; Lin, L.; Feng, X. Highly enantioselective fluorination of unprotected 3-substituted oxindoles: One-step synthesis of BMS 204352 (MaxiPost). J. Org. Chem. 2012, 77, 9148–9155. [Google Scholar] [CrossRef] [PubMed]
- Poikans, J.; Tirzitis, G.; Bisenieks, E.; Uldrikis, J.; Gurevich, V.S.; Mikhailova, I.A.; Duburs, G. The derivatives of 2,6-dimethyl-1,4-dihydroisonicotinic acid and their antiplatelet properties. Eur. J. Med. Chem. 1994, 29, 325–328. [Google Scholar] [CrossRef]
- Dubur, G.Y.; Uldrikis, Y.R. Preparation of 3,5-diethoxycarbonyl-2,6-dimethyl-1,4-dihydroisonicotinic acid and 3,5-diacetyl-2,6-dimethyl-1,4-dihydroisonicotinic acid and their salts. Chem. Heterocycl. Compd. 1972, 5, 762–763. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisenieks, E.; Poikans, J.; Plotniece, A.; Bernotiene, E.; Tsai, W.-B.; Sobolev, A. Sodium N-(3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carbonyl)-l-methioninate. Molbank 2020, 2020, M1148. https://0-doi-org.brum.beds.ac.uk/10.3390/M1148
Bisenieks E, Poikans J, Plotniece A, Bernotiene E, Tsai W-B, Sobolev A. Sodium N-(3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carbonyl)-l-methioninate. Molbank. 2020; 2020(3):M1148. https://0-doi-org.brum.beds.ac.uk/10.3390/M1148
Chicago/Turabian StyleBisenieks, Egils, Janis Poikans, Aiva Plotniece, Eiva Bernotiene, Wei-Bor Tsai, and Arkadij Sobolev. 2020. "Sodium N-(3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carbonyl)-l-methioninate" Molbank 2020, no. 3: M1148. https://0-doi-org.brum.beds.ac.uk/10.3390/M1148