3-(4-(Benzyloxy)-3-methoxyphenyl)-[1,2,4]triazolo[4,3-a]pyridine
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental
3.2. Preparation of N-[(3-Methoxy-4-phenylmethoxyphenyl)methylideneamino]pyridin-2-amine (1)
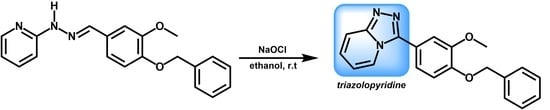
3.3. Preparation of 3-(4-(Benzyloxy)-3-methoxyphenyl)-[1,2,4]triazolo[4,3-a]pyridine (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, G. The chemistry of the triazolopyridines: An update. In Advances in Heterocyclic Chemistry; Academic Press: Cambridge, MA, USA, 2002; Volume 83, pp. 1–70. [Google Scholar] [CrossRef]
- Poormoradkhan Melal, S.; Mahmoodi, N.O. A review of synthetic methods of 1,2,4-triazolopyridines and their therapeutic properties. Results Chem. 2023, 5, 100782. [Google Scholar] [CrossRef]
- Szabó, G.; Károlyi, B.I.; Vaskó, Á.G.; Potor, A.; Vukics, K.; Kapus, G.L.; Fodor, L.; Bobok, A.; Vastag, M.; Bata, I. Identification of Triazolopyridines as Selective α5-GABAAReceptor Negative Allosteric Modulators by a Hybridization Approach. ACS Chem. Neurosci. 2023, 14, 148–158. [Google Scholar] [CrossRef]
- Yang, L.; Bo Xu, W.; Sun, L.; Zhang, C.; Hua Jin, C. SAR Analysis of Heterocyclic Compounds with Monocyclic and Bicyclic Structures as Antifungal Agents. ChemMedChem 2022, 17, e202200221. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, G.; Xia, Z.; Chen, N.; Yang, S.; Li, L. Identification of triazolopyridine derivatives as a new class of AhR agonists and evaluation of anti-psoriasis effect in a mouse model. Eur. J. Med. Chem. 2022, 231, 114122. [Google Scholar] [CrossRef]
- Menet, C.J.; Fletcher, S.R.; Van Lommen, G.; Geney, R.; Blanc, J.; Smits, K.; Jouannigot, N.; Deprez, P.; van der Aar, E.M.; Clement-Lacroix, P.; et al. Triazolopyridines as Selective JAK1 Inhibitors: From Hit Identification to GLPG0634. J. Med. Chem. 2014, 57, 9323–9342. [Google Scholar] [CrossRef] [PubMed]
- Manaithiya, A.; Alam, O.; Sharma, V.; Naim, M.J.; Mittal, S.; Azam, F.; Husain, A.; Sheikh, A.A.; Imran, M.; Khan, I.A. Current Status of Novel Pyridine Fused Derivatives as Anticancer Agents: An Insight into Future Perspectives and Structure Activity Relationship (SAR). Curr. Top. Med. Chem. 2021, 21, 2292–2349. [Google Scholar] [CrossRef]
- Du, W.; Zhang, F.; Yan, P.; Lai, Q.; Li, J.; Zhu, D.; Ye, L. Design, Synthesis, Activity Evaluation and Molecular Docking Study of Novel Janus kinase Inhibitors. ChemistrySelect 2021, 6, 4212–4216. [Google Scholar] [CrossRef]
- Hiranmartsuwan, P.; Wangngae, S.; Nootem, J.; Kamkaew, A.; Daengngern, R.; Wattanathana, W.; Chansaenpak, K. BODIPY-Based Fluorescent Probes for Selective Visualization of Endogenous Hypochlorous Acid in Living Cells via Triazolopyridine Formation. Biosensors 2022, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Chadlaoui, M.; Abarca, B.; Ballesteros, R.; Ramírez de Arellano, C.; Aguilar, J.; Aucejo, R.; García-España, E. Properties of a Triazolopyridine System as a Molecular Chemosensor for Metal Ions, Anions, and Amino Acids. J. Org. Chem. 2006, 71, 9030–9034. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; You, Q.; Lan, J.; Guo, Q.; Li, X.; Xue, Y.; You, J. Cu-catalysed direct C–H (hetero)arylation of [1{,}2{,}4]triazolo[4{,}3-a]pyridine to construct deep-blue-emitting luminophores. Org. Biomol. Chem. 2015, 13, 5372–5375. [Google Scholar] [CrossRef] [PubMed]
- Inturi, S.B.; Kalita, B.; Ahamed, A.J. I2-TBHP-catalyzed one-pot highly efficient synthesis of 4{,}3-fused 1{,}2{,}4-triazoles from N-tosylhydrazones and aromatic N-heterocycles via intermolecular formal 1,3-dipolar cycloaddition. Org. Biomol. Chem. 2016, 14, 11061–11064. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Singh, R.K.; Kant, R. A convenient one-pot synthesis of N-fused 1,2,4-triazoles via oxidative cyclization using chromium (VI) oxide. Synth. Commun. 2019, 49, 22–31. [Google Scholar] [CrossRef]
- Bhatt, A.; Singh, R.K.; Kant, R. Facile one pot synthesis of N-fused 1,2,4-triazoles via oxidative cyclisation using DDQ. ARKIVOC 2018, 2018, 236–247. [Google Scholar] [CrossRef]
- Vidavalur, S.; Nakka, M.; Tadikonda, R.; Rayavarapu, S.; Sarakula, P. A Simple and Efficient Synthesis of 3,4,5-Trisubstituted/N-Fused 1,2,4-Triazoles via Ceric Ammonium Nitrate Catalyzed Oxidative Cyclization of Amidrazones with Aldehydes Using Polyethylene Glycol as a Recyclable Reaction Medium. Synthesis 2015, 47, 517–525. [Google Scholar] [CrossRef]
- Vadagaonkar, K.S.; Murugan, K.; Chaskar, A.C.; Bhate, P.M. A facile and practical one-pot synthesis of [1,2,4]triazolo[4,3-a]pyridines. RSC Adv. 2014, 4, 34056–34064. [Google Scholar] [CrossRef]
- Vadagaonkar, K.S.; Yang, C.-J.; Zeng, W.-H.; Chen, J.-H.; Patil, B.N.; Chetti, P.; Chen, L.-Y.; Chaskar, A.C. Triazolopyridine hybrids as bipolar host materials for green phosphorescent organic light-emitting diodes (OLEDs). Dye. Pigment. 2019, 160, 301–314. [Google Scholar] [CrossRef]
- Reichelt, A.; Falsey, J.R.; Rzasa, R.M.; Thiel, O.R.; Achmatowicz, M.M.; Larsen, R.D.; Zhang, D. Palladium-Catalyzed Chemoselective Monoarylation of Hydrazides for the Synthesis of [1,2,4]Triazolo[4,3-a]pyridines. Org. Lett. 2010, 12, 792–795. [Google Scholar] [CrossRef]
- Thiel, O.R.; Achmatowicz, M.M.; Reichelt, A.; Larsen, R.D. Palladium-Catalyzed Coupling of Aldehyde-Derived Hydrazones: Practical Synthesis of Triazolopyridines and Related Heterocycles. Angew. Chem. Int. Ed. 2010, 49, 8395–8398. [Google Scholar] [CrossRef]
- Nandi, J.; Vaughan, M.; Leon Sandoval, A.; Paolillo, J.; Leadbeater, N. Oxidative Amidation of Amines in Tandem with Transamidation: A Route to Amides Using Visible-Light Energy. J. Org. Chem. 2020, 85, 9219–9229. [Google Scholar] [CrossRef]
- Politano, F.; León Sandoval, A.; Witko, M.L.; Doherty, K.E.; Schroeder, C.M.; Leadbeater, N.E. Nitroxide-Catalyzed Oxidative Amidation of Aldehydes to Yield N-Acyl Azoles Using Sodium Persulfate. Eur. J. Org. Chem. 2022, 2022, e202101239. [Google Scholar] [CrossRef]
- Nandi, J.; Ovian, J.M.; Kelly, C.B.; Leadbeater, N.E. Oxidative Functionalisation of Alcohols and Aldehydes via the Merger of Oxoammonium Cations and Photoredox Catalysis. Org. Biomol. Chem. 2017, 15, 8295–8301. [Google Scholar] [CrossRef]
- León Sandoval, A.; Doherty, K.E.; Wadey, G.P.; Leadbeater, N.E. Solvent- and Additive-Free Oxidative Amidation of Aldehydes Using a Recyclable Oxoammonium Salt. Org. Biomol. Chem. 2022, 20, 2249–2254. [Google Scholar] [CrossRef]
- Zhang, Y.; Teng, H.; Gao, Y.; Afzal, M.W.; Tian, J.; Chen, X.; Tang, H.; James, T.D.; Guo, Y. A general strategy for selective detection of hypochlorous acid based on triazolopyridine formation. Chin. Chem. Lett. 2020, 31, 2917–2920. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doherty, K.E.; León Sandoval, A.; Mercier, E.T.; Leadbeater, N.E. 3-(4-(Benzyloxy)-3-methoxyphenyl)-[1,2,4]triazolo[4,3-a]pyridine. Molbank 2023, 2023, M1694. https://0-doi-org.brum.beds.ac.uk/10.3390/M1694
Doherty KE, León Sandoval A, Mercier ET, Leadbeater NE. 3-(4-(Benzyloxy)-3-methoxyphenyl)-[1,2,4]triazolo[4,3-a]pyridine. Molbank. 2023; 2023(3):M1694. https://0-doi-org.brum.beds.ac.uk/10.3390/M1694
Chicago/Turabian StyleDoherty, Katrina E., Arturo León Sandoval, Ethan T. Mercier, and Nicholas E. Leadbeater. 2023. "3-(4-(Benzyloxy)-3-methoxyphenyl)-[1,2,4]triazolo[4,3-a]pyridine" Molbank 2023, no. 3: M1694. https://0-doi-org.brum.beds.ac.uk/10.3390/M1694