4-(4-Ethoxyphenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazol-3-one
Abstract
:1. Introduction
2. Results and Discussion
2.1. Explanation of Experimental NMR Data for Compound (2)
2.2. Explanation of Experimental NMR Data for Compound (3)
3. Materials and Methods
4. Experimental
4.1. Synthesis of 2-((4-(4-Ethoxyphenyl)-5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)thio)-1-phenylethan-1-one (2)
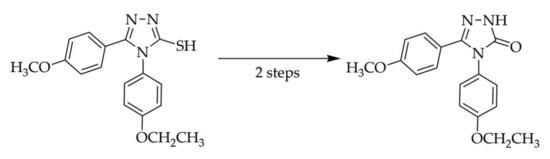
4.2. Synthesis of 4-(4-Ethoxyphenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazol-3-one (3)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferreira, V.F.; da Rocha, D.R.; da Silva, F.C.; Ferreira, P.G.; Boechat, N.A.; Magalhães, J.L. Novel 1H-1,2,3-, 2H-1,2,3-, 1H-1,2,4- and 4H-1,2,4-Triazole Derivatives: A Patent Review (2008–2011). Expert Opin. Ther. Pat. 2013, 23, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüzel, Ş.G.; Çıkla-Süzgün, P. Recent Advances Bioactive 1,2,4-Triazole-3-Thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef] [PubMed]
- Šermukšnytė, A.; Kantminienė, K.; Jonuškienė, I.; Tumosienė, I.; Petrikaitė, V. The Effect of 1,2,4-Triazole-3-Thiol Derivatives Bearing Hydrazone Moiety on Cancer Cell Migration and Growth of Melanoma, Breast, and Pancreatic Cancer Spheroids. Pharmaceuticals 2022, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brahmbhatt, J.G.; Pandya, P.A.; Daraji, D.G.; Patel, H.D.; Rawal, R.M.; Baran, S.K. Design, Synthesis and Biological Evaluation of Novel 5-(4-Chlorophenyl)-4-Phenyl-4H-1,2,4-Triazole-3-Thiols as an Anticancer Agent. J. Mol. Struct. 2021, 1231, 130000. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Farahat, M.M.K.; Awad, L.F.; Balbaa, M.; Yusef, H.; Badawy, M.E.I.; Abd Al Moaty, M.N. New 4-(Arylidene)Amino-1,2,4-Traizole-5-Thiol Derivatives and Their Acyclo Thioglycosides as α-Glucosidase and α-Amylase Inhibitors: Design, Synthesis, and Molecular Modelling Studies. J. Mol. Struct. 2022, 1259, 132733. [Google Scholar] [CrossRef]
- Ihnatova, T.; Kaplaushenko, A.; Frolova, Y.; Pryhlo, E. Synthesis and Antioxidant Properties of Some New 5-Phenethyl-3-Thio-1,2,4-Triazoles. Pharmacia 2021, 68, 129–133. [Google Scholar] [CrossRef]
- Domyati, D.; Zabin, S.A.; Elhenawy, A.A.; Abdelbaset, M. Preparation, Antimicrobial Activity and Docking Study of Vanadium Mixed Ligand Complexes Containing 4-Amino-5-Hydrazinyl-4H-1,2,4-Triazole-3-Thiol and Aminophenol Derivatives. Processes 2021, 9, 1008. [Google Scholar] [CrossRef]
- Arustamyan, Z.S.; Margaryan, R.E.; Aghekyan, A.A.; Panosyan, G.A.; Muradyan, R.E.; Tumajyan, A.E. Synthesis and Anti-Inflammatory Properties of Substituted 5-(Tetrahydro-4-Phenyl-2H-Pyran-4-Yl)-4H-1,2,4-Triazole-3-Thiols. Russ. J. Org. Chem. 2021, 57, 195–202. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Kandeel, M.; Pillay, M.; Deb, P.K.; Abdallah, H.H.; Mahomoodally, M.F.; Chopra, D. Anti-Tubercular Properties of 4-Amino-5-(4-Fluoro-3- Phenoxyphenyl)-4H-1,2,4-Triazole-3-Thiol and Its Schiff Bases: Computational Input and Molecular Dynamics. Antibiotics 2020, 9, 559. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Ashraf, M.; Rehman, J.; Saleem, R.S.Z. Synthesis, in Vitro and in Silico Studies of S-Alkylated 5-(4-Methoxyphenyl)-4-Phenyl-4H-1,2,4-Triazole-3-Thiols as Cholinesterase Inhibitors. Pak. J. Pharm. Sci. 2018, 31, 2697–2708. [Google Scholar]
- Ünver, Y.; Deniz, S.; Çelik, F.; Akar, Z.; Küçük, M.; Sancak, K. Synthesis of New 1,2,4-Triazole Compounds Containing Schiff and Mannich Bases (Morpholine) with Antioxidant and Antimicrobial Activities. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. S3), 89–95. [Google Scholar] [CrossRef]
- Tumosienė, I.; Jonuškienė, I.; Kantminienė, K.; Šiugždaitė, J.; Mickevičius, V.; Beresnevičius, Z.J. Synthesis and Biological Activity of 1,3,4-Oxa(Thia)Diazole, 1,2,4-Triazole-5-(Thio)One and S-Substituted Derivatives of 3-((2-Carboxyethyl)Phenylamino)Propanoic Acid. Res. Chem. Intermed. 2016, 42, 4459–4477. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Sayadi, M. Syntheses of Some Novel and Symmetrical Bis(4-Amino-4H-1,2,4-Triazole-3-Thiols). J. Sulphur. Chem. 2012, 33, 647–652. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Haukka, M.; Soliman, S.M.; Barakat, A.; Boraei, A.T.A.; Aboelmagd, A. Stereoselective Synthesis of New 4-Aryl-5-Indolyl-1,2,4-Triazole S- and N-β-Galactosides: Characterizations, X-Ray Crystal Structure and Hirshfeld Surface Analysis. Crystals 2023, 13, 797. [Google Scholar] [CrossRef]
- Il’inykh, E.S.; Kim, D.G.; Valova, M.S.; Fedorova, O.V. Synthesis and Optical Properties of New S-Derivatives of 5,5′-(1,4-Phenylene)Bis(4H-1,2,4-Triazole-3-Thiol) and 5,5′,5″-(Benzene-1,3,5-Triyl)Tris(4H-1,2,4-Triazole-3-Thiol). Russ. J. Gen. Chem. 2019, 89, 2571–2576. [Google Scholar] [CrossRef]
- Salvatore, R.N.; Smith, R.A.; Nischwitz, A.K.; Gavin, T. A Mild and Highly Convenient Chemoselective Alkylation of Thiols Using Cs2CO3–TBAI. Tetrahedron Lett. 2005, 46, 8931–8935. [Google Scholar] [CrossRef]
- Socea, L.; Barbuceanu, S.; Socea, B.; Draghici, C.; Apostol, T.-V.; Pahontu, E.; Olaru, O. New Heterocyclic Compounds from 1,2,4-Triazoles Class with Potential Cytotoxic Activity. Rev. Chim. 2017, 68, 2503–2508. [Google Scholar] [CrossRef]
- Deligeorgiev, T.; Kaloyanova, S.; Lesev, N.; Vaquero, J.J. An Easy and Fast Ultrasonic Selective S-Alkylation of Hetaryl Thiols at Room Temperature. Ultrason. Sonochem. 2010, 17, 783–788. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S.-B.; Cao, X.; Liu, D.-C.; Shu, B.; Quan, Z.-S. Design, Synthesis and Anticonvulsant Activity Evaluation of Novel 4-(4-Substitutedphenyl)-3-Methyl-1H-1,2,4-Triazol-5(4H)-Ones. Drug Res. 2013, 64, 40–46. [Google Scholar] [CrossRef]
- An, F.; Xuan, X.; Liu, Z.; Bian, M.; Shen, Q.; Quan, Z.; Zhang, G.; Wei, C. Anti-Inflammatory Activity of 4-(4-(Heptyloxy)Phenyl)-2,4-Dihydro-3H-1,2,4-Triazol-3-One via Repression of MAPK/NF-ΚB Signaling Pathways in β-Amyloid-Induced Alzheimer’s Disease Models. Molecules 2022, 27, 5035. [Google Scholar] [CrossRef]
- Ustabaş, R.; Süleymanoğlu, N.; Ünver, Y.; Direkel, Ş. 5-(4-Bromobenzyl)-4-(4-(5-Phenyl-1,3,4-Oxadiazole-2-Yl)Phenyl)-2,4-Dihydro-3H-1,2,4-Triazole-3-One: Synthesis, Characterization, DFT Study and Antimicrobial Activity. J. Mol. Struct. 2020, 1214, 128217. [Google Scholar] [CrossRef]
- Malbec, F.; Milcent, R.; Vicart, P.; Bure, A.M. Synthesis of New Derivatives of 4-Amino-2,4-Dihydro-1,2,4-Triazol-3-One as Potential Antibacterial Agents. J. Heterocycl. Chem. 1984, 21, 1769–1774. [Google Scholar] [CrossRef]
- Sirach, R.R.; Dave, P.N. 3-Nitro-1,2,4-Triazol-5-One (NTO): High Explosive Insensitive Energetic Material. Chem. Heterocycl. Comp. 2021, 57, 720–730. [Google Scholar] [CrossRef]
- Kikugawa, Y.; Yamada, S.; Nagashima, H.; Kaji, K. The Reaction of Substituted Ureas with Sodium Borohydride in Pyridine. Tetrahedron. Lett. 1969, 10, 699–702. [Google Scholar] [CrossRef]
- Molla, E.; Abser, N.; Islam, M. Synthesis and Characterization of Some 4-Aryl Substituted Thiosemicarbazides, N-Alkyloxybenzaldehydes Containing Long Alkyl Chains and Their Corresponding Thiosemicarbazones. Jahangirnagar. Univ. J. Sci. 2018, 41, 31–42. [Google Scholar]
- Siwek, A.; Wujec, M.; Dobosz, M.; Wawrzycka-Gorczyca, I. Study of Direction of Cyclization of 1-Azolil-4-Aryl/Alkyl-Thiosemicarbazides. Heteroat. Chem. 2010, 21, 521–532. [Google Scholar] [CrossRef]
- Pareek, A.K.; Joseph, P.E.; Seth, D.S. Convenient Synthesis, Characterization of Some Novel Substituted 3-Methyl-2-Pyrazoline-5-Ones and Substituted 3,5-Dimethyl Pyrazoles. Orient. J. Chem. 2010, 26, 1467–1471. [Google Scholar]


| HMBC (1H-15N) | HMBC (1H-13C) |
|---|---|
| 174.9 → 7.16 | 193.3 → 8.04 |
| (4-N) (2″-H, 6″-H) | (C = O) (2′′′-H, 6′′′-H) |
| 152.2 → 4.95 | |
| (3-C) (S-CH2) | |
| 155.1 → 7.36 | |
| (5-C) (2′-H, 6′-H) | |
| 160.6 → 3.77 | |
| (4′-C) (O-CH3) | |
| 159.9 → 4.07 | |
| (4″-C) (O-CH2) |
| HSQC (1H-15N) | HMBC (1H-15N) | HMBC (1H-13C) |
|---|---|---|
| 164.4 → 11.98 | 154.1 → 11.98 | 145.8 → 11.98 |
| (2-N) (2-H) | (4-N) (2-H) | (5-C) (2-H) |
| 254.5 → 11.98 | 155.3 → 11.98 | |
| (1-N) (2-H) | (3-C) (2-H) | |
| 154.1 → 7.16 | 145.8 → 7.22 | |
| (4-N) (2″-H, 6″-H) | (5-C) (2′-H, 6′-H) | |
| 160.6 → 3.72 | ||
| (4′-C) (O-CH3) | ||
| 158.7 → 4.03 | ||
| (4″-C) (O-CH2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burcă, I.; Badea, V.; Deleanu, C.; Bercean, V.-N.; Péter, F. 4-(4-Ethoxyphenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazol-3-one. Molbank 2023, 2023, M1705. https://0-doi-org.brum.beds.ac.uk/10.3390/M1705
Burcă I, Badea V, Deleanu C, Bercean V-N, Péter F. 4-(4-Ethoxyphenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazol-3-one. Molbank. 2023; 2023(3):M1705. https://0-doi-org.brum.beds.ac.uk/10.3390/M1705
Chicago/Turabian StyleBurcă, Ion, Valentin Badea, Calin Deleanu, Vasile-Nicolae Bercean, and Francisc Péter. 2023. "4-(4-Ethoxyphenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazol-3-one" Molbank 2023, no. 3: M1705. https://0-doi-org.brum.beds.ac.uk/10.3390/M1705