Reductive Mobilization of Iron from Intact Ferritin: Mechanisms and Physiological Implication
Abstract
:1. Introduction
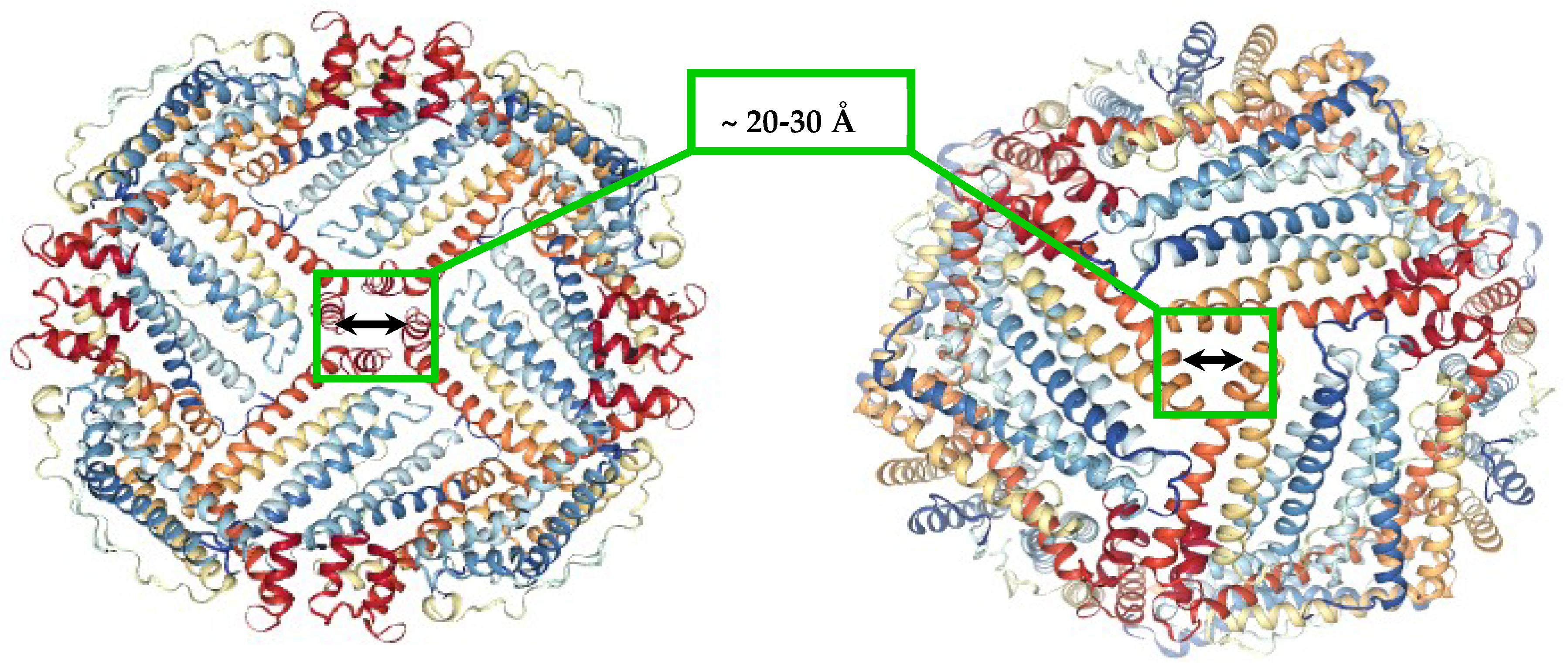
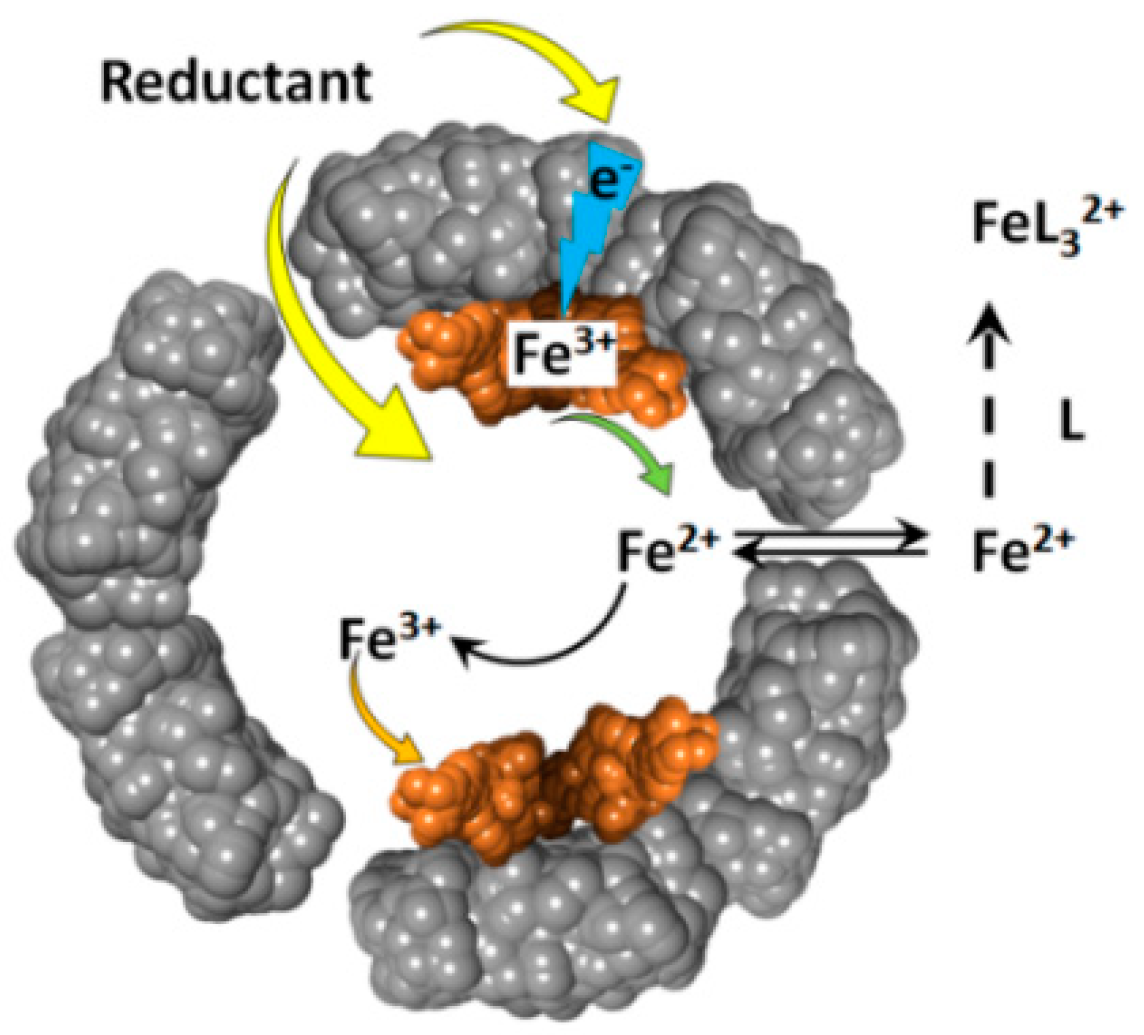
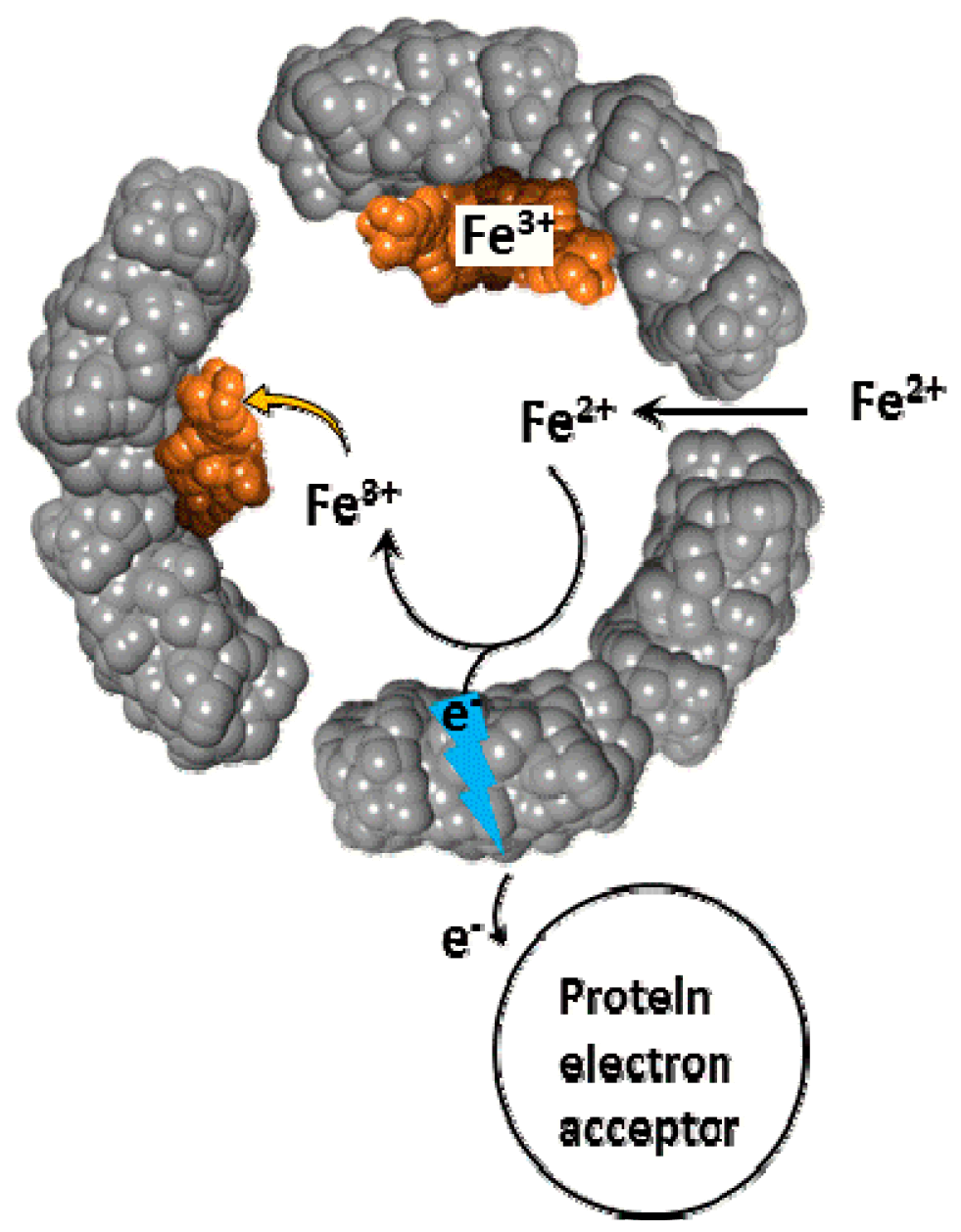
2. Brief Overview of Iron Reductive Mobilization from Intact Ferritin
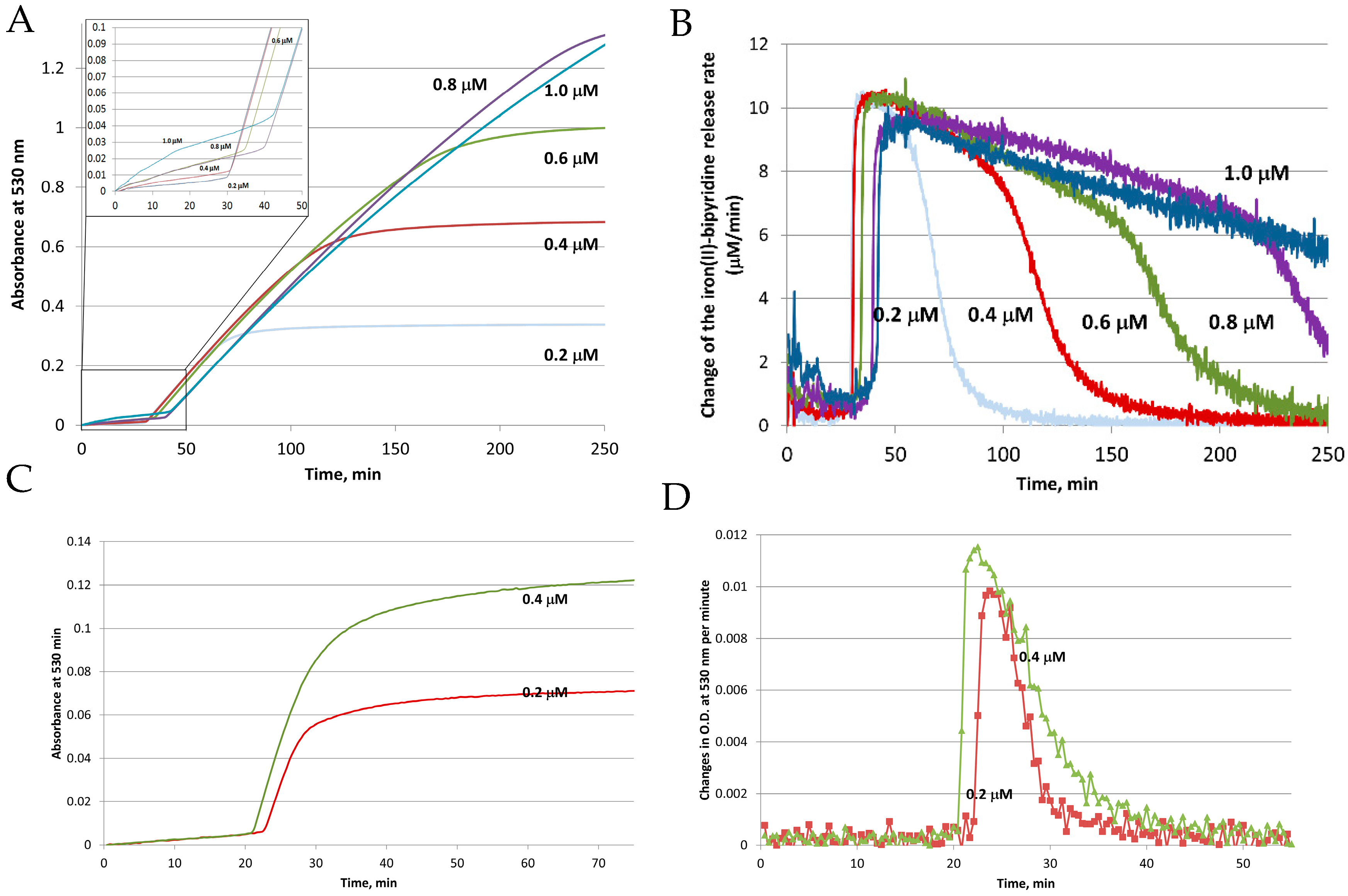
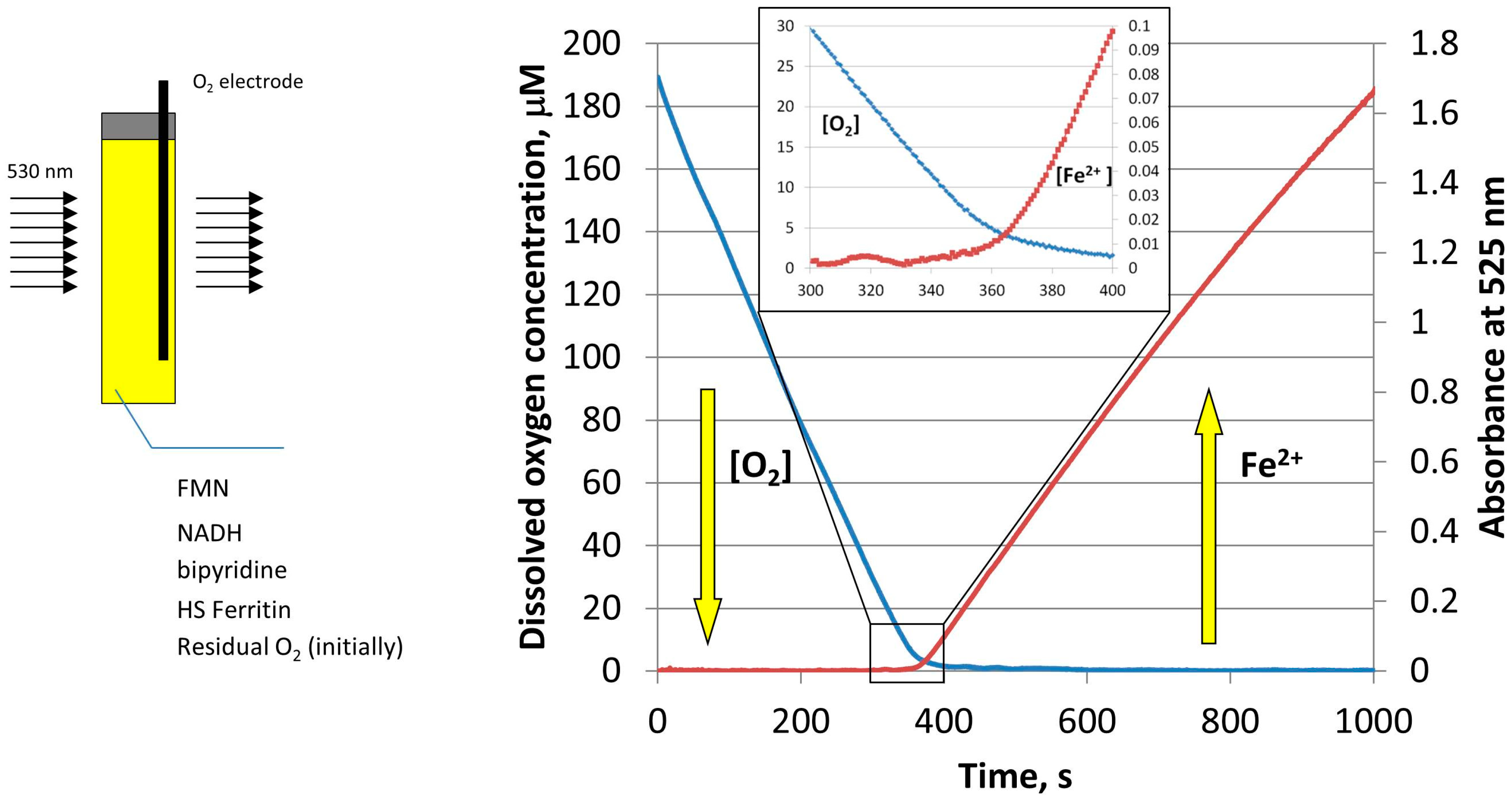
3. Iron Mobilization by the FMN-NADH System
4. Iron Mobilization by Reduced Flavins
5. Iron Mobilization by Other Reducing Agents
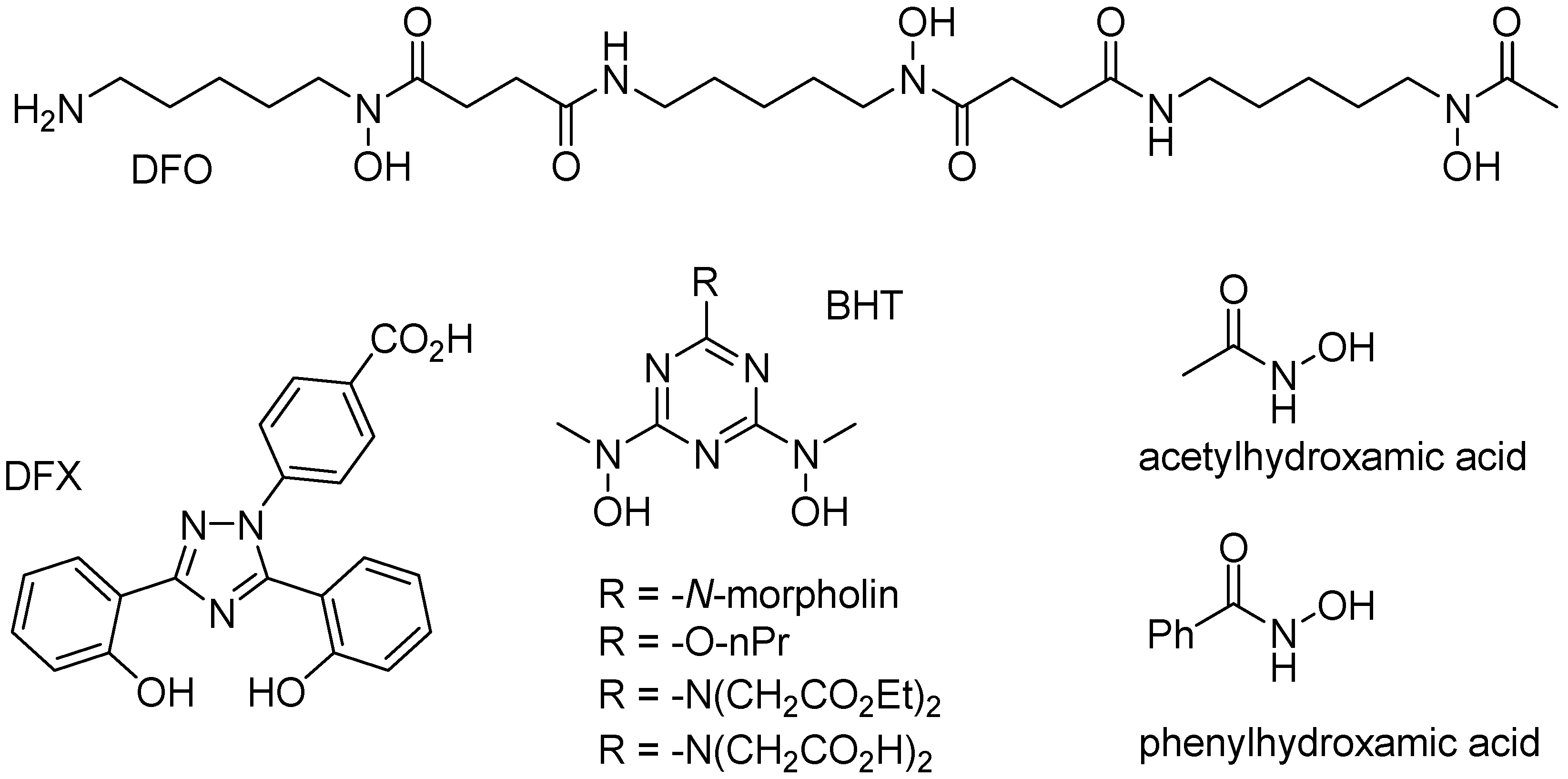
6. Iron Mobilization by Specific Iron(III) Chelating Agents
7. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Britton, R.S.; Leicester, K.L.; Bacon, B.R. Iron toxicity and chelation therapy. Int. J. Hematol. 2002, 76, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Theil, E.C.; Goss, D.J. Living with Iron (and Oxygen): Questions and Answers about Iron Homeostasis. Chem. Rev. 2009, 109, 4568–4579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammack, R. Iron-Sulfur Clusters in Enzymes-Themes and Variations. Adv. Inorg. Chem. 1992, 38, 281–322. [Google Scholar]
- Shaik, S.; Hirao, H.; Kumar, D. Reactivity of high-valent iron-oxo species in enzymes and synthetic reagents: A tale of many states. Acc. Chem. Res. 2007, 40, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Bruijnincx, P.C.A.; van Koten, G.; Gebbink, R. Mononuclear non-heme iron enzymes with the 2-His-1-carboxylate facial triad: Recent developments in enzymology and modeling studies. Chem. Soc. Rev. 2008, 37, 2716–2744. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.F.; Bencze, K.Z.; Stemmler, T.L.; Philpott, C.C. A cytosolic iron chaperone that delivers iron to ferritin. Science 2008, 320, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F. The iron redox and hydrolysis chemistry of the ferritins. BBA-Gen. Subj. 2010, 1800, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. BBA-Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Chasteen, N.D. Ferritin. Uptake, storage, and release of iron. Met. Ions Biol. Syst. 1998, 35, 479–514. [Google Scholar] [PubMed]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. BBA-Gen. Subj. 2009, 1790, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Mehlenbacher, M.; Poli, M.; Arosio, P.; Santambrogio, P.; Levi, S.; Chasteen, N.D.; Bou-Abdallah, F. Iron oxidation and core formation in recombinant heteropolymeric human ferritins. Biochemistry 2017, 56, 3900–3912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Bou-Abdallah, F.; Arosio, P.; Levi, S.; Janus-Chandler, C.; Chasteen, N.D. Multiple pathways for mineral core formation in mammalian apoferritin. The role of hydrogen peroxide. Biochemistry 2003, 42, 3142–3150. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F.; Yang, H.; Awomolo, A.; Cooper, B.; Woodhall, M.R.; Andrews, S.C.; Chasteen, N.D. Functionality of the three-site ferroxidase center of Escherichia coli bacterial ferritin (EcFtnA). Biochemistry 2014, 53, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F.; Zhao, G.; Biasiotto, G.; Poli, M.; Arosio, P.; Chasteen, N.D. Facilitated diffusion of iron(II) and dioxygen substrates into human H-chain ferritin. A fluorescence and absorbance study employing the ferroxidase center substitution Y34W. J. Am. Chem. Soc. 2008, 130, 17801–17811. [Google Scholar] [CrossRef] [PubMed]
- Theil, E.C. Ferritin: The protein nanocage and iron biomineral in health and in disease. Inorg. Chem. 2013, 52, 12223–12233. [Google Scholar] [CrossRef] [PubMed]
- Honarmand Ebrahimi, K.; Hagedoorn, P.-L.; Hagen, W.R. Unity in the Biochemistry of the iron-storage proteins ferritin and bacterioferritin. Chem. Rev. 2014, 115, 295–326. [Google Scholar] [CrossRef] [PubMed]
- Haikarainen, T.; Papageorgiou, A.C. Dps-like proteins: Structural and functional insights into a versatile protein family. Cell. Mol. Life Sci. 2010, 67, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Zeth, K. Dps biomineralizing proteins: Multifunctional architects of nature. Biochem. J. 2012, 445, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M. Bacterioferritin: Structure, Dynamics, and Protein-Protein Interactions at Play in Iron Storage and Mobilization. Acc. Chem. Res. 2017, 50, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Eshelman, K.; Yao, H.; Hewage, A.N.D. P.; Deay, J.J.; Chandler, J.R.; Rivera, M. Inhibiting the BfrB: Bfd interaction in Pseudomonas aeruginosa causes irreversible iron accumulation in bacterioferritin and iron deficiency in the bacterial cytosol. Metallomics 2017, 9, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Weeratunga, S.K.; Gee, C.E.; Lovell, S.; Zeng, Y.; Woodin, C.L.; Rivera, M. Binding of Pseudomonas aeruginosa apobacterioferritin-associated ferredoxin to bacterioferritin B promotes heme mediation of electron delivery and mobilization of core mineral iron. Biochemistry 2009, 48, 7420–7431. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C. Mobilization of stored iron in mammals: A review. Nutrients 2013, 5, 4022–4050. [Google Scholar] [CrossRef] [PubMed]
- Melman, G.; Bou-Abdallah, F.; Vane, E.; Maura, P.; Arosio, P.; Melman, A. Iron release from ferritin by flavin nucleotides. BBA-Gen. Subj. 2013, 1830, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F.; McNally, J.; Liu, X.X.; Melman, A. Oxygen catalyzed mobilization of iron from ferritin by iron(III) chelate ligands. Chem. Commun. 2011, 47, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.E.; Wilkinson, T.; Arosio, P.; Melman, A.; Bou-Abdallah, F. Effect of chaotropes on the kinetics of iron release from ferritin by flavin nucleotides. BBA-Gen. Subj. 2017, 1861, 3257–3262. [Google Scholar] [CrossRef] [PubMed]
- Watt, R.K.; Hilton, R.J.; Graff, D.M. Oxido-reduction is not the only mechanism allowing ions to traverse the ferritin protein shell. BBA-Gen. Subj. 2010, 1800, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Kidane, T.Z.; Sauble, E.; Linder, M.C. Release of iron from ferritin requires lysosomal activity. Am. J. Physiol. Cell Physiol. 2006, 291, C445–C455. [Google Scholar] [CrossRef] [PubMed]
- La, A.; Nguyen, T.; Tran, K.; Sauble, E.; Tu, D.; Gonzalez, A.; Kidane, T.Z.; Soriano, C.; Morgan, J.; Doan, M. Mobilization of iron from ferritin: New steps and details. Metallomics 2018, 10, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Truty, J.; Malpe, R.; Linder, M.C. Iron prevents ferritin turnover in hepatic cells. J. Biol. Chem. 2001, 276, 48775–48780. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Spencer, R.; Walsh, C. Mechanism and kinetics of iron release from ferritin by dihydroflavins and dihydroflavin analogs. Biochemistry 1978, 17, 4011–4017. [Google Scholar] [CrossRef] [PubMed]
- Sirivech, S.; Frieden, E.; Osaki, S. The release of iron from horse spleen ferritin by reduced flavins. Biochem. J. 1974, 143, 311–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, R.F.; Grabill, T.W.; Petrovich, R.M. Reductive release of ferritin iron: A kinetic assay. Anal. Biochem. 1988, 174, 17–22. [Google Scholar] [CrossRef]
- Sakurai, K.; Nabeyama, A.; Fujimoto, Y. Ascorbate-mediated iron release from ferritin in the presence of alloxan. Biometals 2006, 19, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Funk, F.; Lenders, J.P.; Crichton, R.R.; Schneider, W. Reductive mobilisation of ferritin iron. FEBS J. 1985, 152, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Bolann, B.J.; Ulvik, R.J. Release of iron from ferritin by xanthine oxidase. Role of the superoxide radical. Biochem. J. 1987, 243, 55–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, H.P.; Winterbourn, C.C. The superoxide-dependent transfer of iron from ferritin to transferrin and lactoferrin. Biochem. J. 1988, 256, 923–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, M.S.; Tourillon, G.; Sayers, D.E.; Theil, E.C. Rapid reduction of iron in horse spleen ferritin by thioglycolic acid measured by dispersive X-ray absorption spectroscopy. Biol. Met. 1990, 3, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Jepkorir, G.; Lovell, S.; Nama, P.V.; Weeratunga, S.; Battaile, K.P.; Rivera, M. Two Distinct Ferritin-like Molecules in Pseudomonas aeruginosa: The Product of the bfrA Gene Is a Bacterial Ferritin (FtnA) and Not a Bacterioferritin (Bfr). Biochemistry 2011, 50, 5236–5248. [Google Scholar] [CrossRef] [PubMed]
- Orino, K.; Kamura, S.; Natsuhori, M.; Yamamoto, S.; Watanabe, K. Two pathways of iron uptake in bovine spleen apoferritin dependent on iron concentration. Biometals 2002, 15, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Arosio, P.; Levi, S.; Chasteen, N.D. Ferroxidase kinetics of human liver apoferritin, recombinant H-chain apoferritin, and site-directed mutants. Biochemistry 1993, 32, 9362–9369. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.L.; Franz, K.J. Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, W.; Theil, E.C. Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proc. Natl. Acad. Sci. USA 2003, 100, 3653–3658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, Q.H.; Hastings, J.W. The oxidation of reduced flavin mononucleotide by molecular oxygen. Biochem. J. 1962, 83, 368–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topham, R.; Goger, M.; Pearce, K.; Schultz, P. The mobilization of ferritin iron by liver cytosol. A comparison of xanthine and NADH as reducing substrates. Biochem. J. 1989, 261, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theil, E.C.; Tosha, T.; Behera, R.K. Solving Biology’s Iron Chemistry Problem with Ferritin Protein Nanocages. Acc. Chem. Res. 2016, 49, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.D.; Jacobs, D.; Frankel, R.B. Redox reactivity of bacterial and mammalian ferritin: Is reductant entry into the ferritin interior a necessary step for iron release? Proc. Natl. Acad. Sci. USA 1988, 85, 7457–7461. [Google Scholar] [CrossRef] [PubMed]
- Theil, E.C.; Liu, X.S.; Tosha, T. Gated pores in the ferritin protein nanocage. Inorg. Chim. Acta 2008, 361, 868–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.S.; Patterson, L.D.; Miller, M.J.; Theil, E.C. Peptides selected for the protein nanocage pores change the rate of iron recovery from the ferritin mineral. J. Biol. Chem. 2007, 282, 31821–31825. [Google Scholar] [CrossRef] [PubMed]
- Carmona, U.; Li, L.; Zhang, L.; Knez, M. Ferritin light-chain subunits: Key elements for the electron transfer across the protein cage. Chem. Commun. 2014, 50, 15358–15361. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, N.; Pane, F.; Messori, L.; Amoresano, A.; Merlino, A. Cisplatin encapsulation within a ferritin nanocage: A high-resolution crystallographic study. Chem. Commun. 2016, 52, 4136–4139. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bai, G.; Yang, S.; Yang, R.; Zhao, G.; Xu, C.; Leung, W. Encapsulation of curcumin in recombinant human H-chain ferritin increases its water-solubility and stability. Food Res. Int. 2014, 62, 1147–1153. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, C.; Chen, L.; Bai, G.; Zhao, G.; Xu, C. Encapsulation of anthocyanin molecules within a ferritin nanocage increases their stability and cell uptake efficiency. Food Res. Int. 2014, 62, 183–192. [Google Scholar] [CrossRef]
- Chen, L.; Bai, G.; Yang, R.; Zang, J.; Zhou, T.; Zhao, G. Encapsulation of beta-carotene within ferritin nanocages greatly increases its water-solubility and thermal stability. Food Chem. 2014, 149, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.M.; Svistunenko, D.A.; Lawson, T.L.; Hemmings, A.M.; Moore, G.R.; Le Brun, N.E. Three Aromatic Residues are Required for Electron Transfer during Iron Mineralization in Bacterioferritin. Angew. Chem. Int. Ed. Engl. 2015, 54, 14763–14767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, J.M.; Svistunenko, D.A.; Moore, G.R.; Le Brun, N.E. Tyr25, Tyr58 and Trp133 of Escherichia coli bacterioferritin transfer electrons between iron in the central cavity and the ferroxidase centre. Metallomics 2017, 9, 1421–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koochana, P.K.; Mohanty, A.; Das, S.; Subhadarshanee, B.; Satpati, S.; Dixit, A.; Sabat, S.C.; Behera, R.K. Releasing iron from ferritin protein nanocage by reductive method: The role of electron transfer mediator. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Bienfait, H.F.; Van Den Briel, M.L. Rapid mobilization of ferritin iron by ascorbate in the presence of oxygen. BBA-Gen. Subj. 1980, 631, 507–510. [Google Scholar] [CrossRef]
- Badu-Boateng, C.; Naftalin, R.J. Ascorbate and ferritin interactions: Consequences for iron release in vitro and in vivo and implications for inflammation. Free Radic. Biol. Med. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Badu-Boateng, C.; Pardalaki, S.; Wolf, C.; Lajnef, S.; Peyrot, F.; Naftalin, R.J. Labile iron potentiates ascorbate-dependent reduction and mobilization of ferritin iron. Free Radic. Biol. Med. 2017, 108, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Gardner, P.R. Aconitase: Sensitive target and measure of superoxide. Methods Enzymol. 2002, 349, 9–23. [Google Scholar] [PubMed]
- Hynes, M.J.; Coinceanainn, M.O. Investigation of the release of iron from ferritin by naturally occurring antioxidants. J. Inorg. Biochem. 2002, 90, 18–21. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, V.; Rao, G.S. Release of iron from ferritin by 1, 2, 4-benzenetriol. Chem. Biol. Interact. 1995, 96, 103–111. [Google Scholar] [CrossRef]
- Dangles, O.; Fargeix, G.; Dufour, C. One-electron oxidation of quercetin and quercetin derivatives in protic and non protic media. J. Chem. Soc. Perkin Trans. 2 1999, 1387–1396. [Google Scholar] [CrossRef]
- Bennett, B.D.; Kimball, E.H.; Gao, M.; Osterhout, R.; Van Dien, S.J.; Rabinowitz, J.D. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 2009, 5, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Chaston, T.B.; Richardson, D.R. Iron Chelators for the treatment of iron overload disease: Relationship between structure, redox activity, and toxicity. Am. J. Hematol. 2003, 73, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Galvez, N.; Ruiz, B.; Cuesta, R.; Colacio, E.; Dominguez-Vera, J.M. Release of iron from ferritin by aceto-and benzohydroxamic acids. Inorg. Chem. 2005, 44, 2706–2709. [Google Scholar] [CrossRef] [PubMed]
- Galvez, N.; Fernandez, B.; Sanchez, P.; Cuesta, R.; Ceolin, M.; Clemente-Leon, M.; Trasobares, S.; Lopez-Haro, M.; Calvino, J.J.; Stephan, O.; et al. Comparative structural and chemical studies of ferritin cores with gradual removal of their iron contents. J. Am. Chem. Soc. 2008, 130, 8062–8068. [Google Scholar] [CrossRef] [PubMed]
- Netto, L.E.S.; Da Costa Ferreira, A.M.; Augusto, O. Iron(III) binding in DNA solutions: Complex formation and catalytic activity in the oxidation of hydrazine derivatives. Chem. Biol. Interact. 1991, 79, 1–14. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou-Abdallah, F.; Paliakkara, J.J.; Melman, G.; Melman, A. Reductive Mobilization of Iron from Intact Ferritin: Mechanisms and Physiological Implication. Pharmaceuticals 2018, 11, 120. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040120
Bou-Abdallah F, Paliakkara JJ, Melman G, Melman A. Reductive Mobilization of Iron from Intact Ferritin: Mechanisms and Physiological Implication. Pharmaceuticals. 2018; 11(4):120. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040120
Chicago/Turabian StyleBou-Abdallah, Fadi, John J. Paliakkara, Galina Melman, and Artem Melman. 2018. "Reductive Mobilization of Iron from Intact Ferritin: Mechanisms and Physiological Implication" Pharmaceuticals 11, no. 4: 120. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040120




