Dissipative Particle Dynamics Investigation of the Transport of Salicylic Acid through a Simulated In Vitro Skin Permeation Model
Abstract
:1. Introduction
2. Results
2.1. Experimental Results
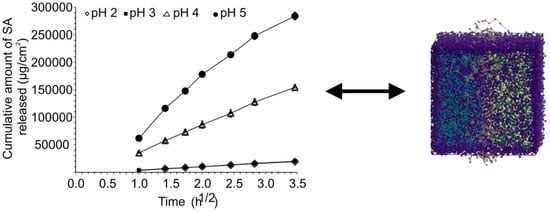
2.1.1. Solubility and Release Studies
2.1.2. Membrane Morphology
2.2. Computational Studies
2.2.1. AA-MD Computational Studies—Interaction Parameters
2.2.2. AA-MD—The Choice of the Polymer Chain Length
2.2.3. DPD Simulations
3. Discussion
3.1. Release, Partition and Morphology Studies
3.2. Computational Studies
3.2.1. AA-MD Computational Studies—Chain Length and χij
3.2.2. DPD Computational Studies
4. Materials and Methods
4.1. Materials
4.2. Experimental Methods
4.2.1. Aqueous Phase Preparation
4.2.2. Solubility Determination of Salicylic Acid
4.2.3. Determination of Percentage Ionized Salicylic Acid
4.2.4. In Vitro Release Study
4.2.5. Statistical Analysis
4.2.6. Degree of Swelling
4.2.7. Scanning Electron Microscope (SEM)
4.2.8. HPLC-UV method
4.3. Computational Modelling Methods
4.3.1. All-Atomistic Molecular Dynamics (AA-MD) Theory
4.3.2. AA-MD Methodology
4.3.3. Dissipative Particle Dynamics (DPD) Simulation Theory
4.3.4. DPD Methodology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiedersberg, S.; Guy, R.H. Transdermal drug delivery: 30+ years of war and still fighting. J. Control. Release 2014, 190, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.F.; Rouse, J.J.; Anderson, F.D.; Eccleston, G.E. The relevance of polymeric synthetic membranes in topical formulation assessment and drug diffusion. Arch. Pharmacal. Res. 2012, 35, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.F.; Rouse, J.J.; Anderson, F.D.; Meidan, V.; Eccleston, G.E. Validation of a static Franz diffusion cell system for in vitro permeation studies. AAPS PharmSciTech 2010, 11, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.L.; Shah, V.P.; Tenjarla, S.N.; Corbo, N.; DeMagistris, D.; Feldmann, T.G.; Franz, T.J.; Miran, D.R.; Pearce, D.M.; Sequiera, J.A.; et al. Assessment of value and applications of in vitro testing of topical dermatological drug products. Pharm. Res. 1999, 16, 1325–1330. [Google Scholar] [CrossRef]
- Choi, S.H.; Wang, Y.; Conti, D.S.; Raney, S.G.; Delvadia, R.; Leboeuf, A.A.; Witzmann, K. Generic drug device combination products: Regulatory and scientific considerations. Int. J. Pharm. 2018, 544, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In vitro-in vivo correlation in skin permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Sugibayashi, K.; Todo, H.; Oshikaza, T.; Owada, Y. Mathematical model to predict skin concentration of drugs: Towards utilization of silicone membrane to predict skin concentration of drugs as an animal testing alternative. Pharm. Res. 2010, 27, 134–142. [Google Scholar] [CrossRef]
- Franz, T.J. Percutaneous adsorption on the relevance of in vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef]
- Houk, J.; Guy, R.H. Membrane models for skin penetration studies. Chem Rev. 1988, 88, 455–471. [Google Scholar] [CrossRef]
- Waters, L.J. Recent developments in skin mimic systems to predict transdermal permeation. Curr. Pharm. Des. 2015, 21, 2725–2732. [Google Scholar] [CrossRef]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Enhanced permeation of fentanyl from supersaturated solutions in a model membrane. Int. J. Pharm. 2011, 407, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Maitani, Y.; Shimada, K.; Nagai, T. l-menthol, oleic acid and lauricidin in absorption enhancement of free and sodium salicylic acid of diclofenac using ethanol treated silicone membrane as model for skin. Chem. Pharm. Bull. 1996, 44, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kotzé, M.; Otto, A.; Jordaan, A.; du Plessis, J. Whey protein/polysaccharide-stabilized oil powders for topical application—Release and transdermal delivery of salicylic acid from oil powders compared to redispersed powders. AAPSPharmSciTech 2015, 16, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, S.; Arrelano, A.; Ygartua, P.; Martin, C. In vitro percutaneous absorption of piroxicam through synthetic membranes and abdominal rat skin. Pharm. Acta Helvet. 1996, 71, 141–146. [Google Scholar] [CrossRef]
- López-Chávez, E.M.; Martínez-Magadán, J.M.; Oviedo-Roa, R.; Guzmán, J.; Ramírez-Salgado, J.; Martínez-Cruz, J. Molecular modeling of ion-conductivity in chitosan membranes. Polymer 2005, 46, 7519–7527. [Google Scholar] [CrossRef]
- Dureja, H.; Tiwary, A.K.; Gupta, S. Simulation of skin permeability in chitosan membrane. Int. J. Pharm. 2001, 213, 193–198. [Google Scholar] [CrossRef]
- Clément, P.; Laugel, C.; Marty, J.P. Influence on three synthetic membranes on the release of caffeine from concentrated W/O emulsions. J. Control. Release 2000, 66, 243–254. [Google Scholar] [CrossRef]
- López-Chávez, E.M.; Martinez-Magadán, J.M.; Oviedo-Roa, R.; Guzmán, J.; Ramírez-Saldago, J.; Marín-Cruz, J. Molecular modeling and simulation of ion conductivity in chitosan membranes. Polymer 2005, 18, 7519–7527. [Google Scholar] [CrossRef]
- Yamane, C.; Miyamoto, H.; Hayakawa, D.; Ueda, K. Folded-chain structure of cellulose II suggested by molecular dynamics simulation. Carbohydr. Res. 2013, 379, 30–37. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Young, R.J.; Davies, G.R. Modeling crystal and molecular deformation in regenerated cellulose fibers. Biomacromolecules 2005, 6, 507–513. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, W.; Xia, J.; Xiao, H. Molecular dynamics study of the structure and performance of simple and double bases propellants. J. Hazard. Mater. 2008, 156, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Milewski, M.; Stinchcomb, A.L. Estination of maximum transdermal flux of nonionized xenobiotics from basic physicochemical determinants. Mol. Pharm. 2012, 9, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Kedem, O.; Katchalsky, A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim. Biophys. Acta 1958, 27, 229–246. [Google Scholar] [CrossRef]
- Matuszak, D.; Aranovich, G.L.; Donohue, M.D. Thermodynamic driving force for molecular diffusion—Lattice density theory functional theory predictions. J. Non-Equilib. Thermodyn. 2006, 31, 355–384. [Google Scholar] [CrossRef]
- Ghonaim, H.M.; Periamy, N.; Nagarajan, N.; Massimo, G.; Anwar, J. Towards a simplified model membrane of skin lipids: Preparation and characterization of a ternary lipid structure. Int. J. Pharm. Pharm. Sci. 2014, 6, 148–152. [Google Scholar]
- Luo, L.; Patel, A.; Sinko, B.; Bell, M.; Wibawa, J.; Hadgraft, J.; Lane, M.E. A comparative study of the in vitro permeation of ibuprofen in mammalian skin, the PAMPA model and silicone membrane. Int. J. Pharm. 2016, 30, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kunst, A.; Lee, G. Release and skin permeation of scopolamine from thin polymer films in relation to thermodynamic activity. J. Pharm. Sci. 2016, 105, 1496–1500. [Google Scholar] [CrossRef] [PubMed]
- Trasi, N.S.; Taylor, L.S. Thermodynamics of highly supersaturated aqueous solutions of poorly water-soluble drugs—Impact of a second drug on the solution phase behavior and implications for combination products. J. Pharm. Sci. 2015, 104, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Brain, K.R.; Walters, K.A.; Watkinson, A.C. Methods for studying percutaneous. In Dermatological and Transdermal Formulations; Walters, K.A., Ed.; Marcel Dekker AG: New York, NY, USA, 2002; pp. 197–270. [Google Scholar]
- Selzer, D.; Abdel-Mottaleb, M.M.A.; Hahn, T.; Schaefer, U.F.; Neumann, D. Finite and infinite dosing: Difficulties in measurements, evaluations and predictions. Adv. Drug Deliv. Rev. 2013, 65, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Bunge, A.L.; Persichetti, J.M.; Payan, J.P. Explaining skin permeation of 2-butoxyethanol from neat and aqueous solutions. Int. J. Pharm. 2012, 435, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Kalia, Y.N.; Guy, R.H. Modeling transdermal drug release. Adv Drug Deliv Rev. 2001, 48, 159–172. [Google Scholar] [CrossRef]
- Fernandes, M.; Simon, L.; Loney, N.W. Mathematical modeling of transdermal drug-delivery systems: Analysis and applications. J. Memb. Sci. 2005, 256, 184–192. [Google Scholar] [CrossRef]
- Simovic, P.; Skundric, I.; Pajic-Lijakovic, I.; Ristic, K.; Medovic, A.; Tasić, G. Mathematical model of gentamicin sulfate release from a bioactive textile material and a transdermal material system under in vitro conditions. J. Appl. Polym. Sci. 2010, 117, 1424–1430. [Google Scholar]
- Paloncýová, M.; Vávrová, K.; Sovová, Z.; DeVane, R.; Otyepka, M.; Berka, K. Structural changes in ceramide bilayers rationalize increased permeation through stratum corneum models with shorter acyl tails. J. Phys. Chem. B 2015, 119, 9811–9819. [Google Scholar] [CrossRef]
- Dias, M.; Hadgraft, J.; Lane, M.E. Influence of membrane-solvent-solute interactions on solute permeation in model membranes. Int. J. Pharm. 2007, 336, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, Y.; Waters, L.J.; Barber, C. Solvent selection effects on the transport of compounds through silicone membranes. Coll. Surf. A Physicochem. Eng. Asp. 2014, 458, 96–100. [Google Scholar] [CrossRef]
- Smith, J.C.; Irwin, W.J. Ionisation and the effect of absorption enhancers on transport of salicylic acid through silastic rubber and human skin. Int. J. Pharm. 2007, 210, 69–82. [Google Scholar] [CrossRef]
- Hoogerbrugge, P.J.; Koelman, J.V.M.A. Simulating microscopic hydrodynamic phenomena with dissipative particle dynamics. Europhys. Lett. 1992, 19, 155–160. [Google Scholar] [CrossRef]
- Koelman, J.V.M.A.; Hoogerbrugge, P.J. Dynamic simulations of hard-sphere suspensions under steady shear. Europhys. Lett. 1993, 21, 363–368. [Google Scholar] [CrossRef]
- Solano Canchaya, J.G.; Dequidt, A.; Goujon, F.; Malfreyt, P. Development of DPD coarse-grained models: From bulk to interfacial properties. J. Chem. Phys. 2016, 145, 054107. [Google Scholar] [CrossRef]
- Ding, H.M.; Tian, W.D.; Ma, Y.Q. Designing nanoparticle translocation through membranes by computer simulations. ACS Nano 2012, 6, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.T.; Yu, X. Charged dendrimers on lipid bilayer membranes: Insight through dissipative particle dynamics simulations. Macromolecules 2009, 42, 6277–6283. [Google Scholar] [CrossRef]
- Gao, L.; Shillock, J.; Lipowsky, R. Improved dissipative particle dynamics simulations of lipid bilayers. J. Chem. Phys. 2007, 126, 01B602. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Shamra, J. Application of DPD in the design of polymeric nano-micelles as drug carriers. J. Mol. Graph. Model. 2016, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, B.; Li, Y.; Liu, Y.; Liu, Q.; Jiang, H.; He, Z.; Zhao, Y.; Sun, J. Experimental observations and dissipative particle dynamics simulations on microstructures of pH-sensitive polymer containing amorphous solid dispersions. Int. J. Pharm. 2017, 517, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Otto, D.P.; Otto, A.; de Villiers, M.M. Experimental and mesoscale computational dynamics studies of the relationship between solubility and release of quercetin from PEG solid dispersions. Int. J. Pharm. 2013, 456, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, M.; Wennberg, C.L.; Naranqifard, A.; Lindahl, E.; Norlén, L. Predicting drug permeability through skin using molecular dynamics simulation. J. Control. Release. 2018, 10, 269–279. [Google Scholar] [CrossRef]
- Das, C.; Olmsted, P.D.; Noro, M.G. Water permeation through stratum corneum lipid bilayers from atomistic simulations. Soft Matter 2009, 5, 4549–4555. [Google Scholar] [CrossRef]
- Gupta, R.; Sridhar, D.B.; Rai, B. Molecular dynamics simulation study of permeation of molecules through skin lipid layer. J. Phys. Chem. B 2016, 120, 8987–8996. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M.; Jarowski, C. Effect of diffusion layer pH and solubility on the dissolution Rate of pharmaceutical acids and their sodium salts. II: Salicylic acid, theophylline, and benzoic acid. J. Pharm. Sci. 1985, 74, 148–154. [Google Scholar]
- Higuchi, T. Physical chemical analysis of percutaneous absorption process from creams and ointments. J. Soc. Cosmet. Chem. 1960, 11, 85–97. [Google Scholar]
- Uragami, T.; Tamura, M.; Sugihara, M. Studies on syntheses and permeabilities of special polymer membranes—IV. Permeation characteristics of cellulose nitrate membranes and cellulose membranes. Angew. Makromol. Chem. 1978, 68, 213–217. [Google Scholar]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Asman, G.; Sanli, O.; Tuncel, D. pH- and temperature-sensitive in vitro release of salicylic acid through poly(vinyl alcohol-g-acrylamide) membranes. J. Appl. Polym. Sci. 2008, 107, 3005–3012. [Google Scholar] [CrossRef]
- Asman, G.; Sanli, O.; Tuncel, D. In vitro release of salicylic acid through poly(vinyl alcohol-g-itaconic acid) membranes. J. Appl. Polym. Sci. 2008, 107, 3291–3299. [Google Scholar] [CrossRef]
- Pires, R.F.; Franco, M.R. Solubility of salicylic acid in water + salicylic acid (NaCl, KCl, NaBr, Na2SO4 and K2SO4) at 293.5–313.3 K. Fluid Phase Equilibria 2012, 330, 48–51. [Google Scholar] [CrossRef]
- Bhattachar, S.N.; Deschenes, L.A.; Wesley, J.A. Solubility: It is not just for physical chemists. Drug Discov. Today 2006, 11, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Vertzoni, M.; Goumas, K.; Reppas, C. Estimating drug solubility in the gastrointestinal tract. Adv. Drug Deliv. Rev. 2007, 59, 591–602. [Google Scholar] [CrossRef]
- Völgyi, G.; Baka, E.; Box, K.J.; Comer, J.E.A.; Takács-Novák, K. Study of the pH-dependent solubility of organic bases. Revisit of Henderson-Hasselbalch relationship. Anal. Chim. Acta 2010, 673, 40–46. [Google Scholar] [CrossRef]
- Zugenmair, P. History of cellulose research. In Crystalline Cellulose and Cellulose Derivatives; Springer: Berlin, Germany, 2000; pp. 7–51. [Google Scholar]
- Lee, C.K.; Kim, E.J.; Jun, J.H. Determination of relative reactivities of free hydroxyl groups in ß-cyclodextrins, amylose and cellulose by reductive-cleavage method. Bull. Korean Chem. Soc. 1999, 20, 1153–1158. [Google Scholar]
- Ravve, A. Priniciples of Polymer Chemistry, 3rd ed.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; 809p. [Google Scholar]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications—Overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Tucker, A.K.; Stevens, M.J. Study of the polymer length dependence of the single chain transition temperature in syndiotactic poly(N-isopropyacrylamide) oligomers in water. Macromolecules 2012, 45, 6697–6703. [Google Scholar] [CrossRef]
- Otto, D.P.; de Villiers, M.M. The experimental evaluation and molecular dynamics simulation of a heat-enhanced transdermal delivery system. AAPS PharmSciTech 2013, 14, 111–120. [Google Scholar] [CrossRef]
- Posocco, P.; Fermeglia, M.; Pricl, S. Morphology prediction of block copolymers for drug delivery by mesoscale simulations. J. Mater. Chem. 2010, 20, 7742–7753. [Google Scholar] [CrossRef]
- Scocchi, G.; Posocco, P.; Fermeglia, M.; Pricl, S. Polymer-clay nanocomposites: A multiscale molecular modeling approach. J. Phys. Chem. B 2007, 111, 2143–2151. [Google Scholar] [CrossRef]
- Andersen, H.C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant-temperature molecular-dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Zhang, M.; Choi, P.; Sundararaj, U. Molecular dynamics and thermal analysis of anomalous thermodynamic behaviour of poly(ether imide)/polycarbonate blends. Polymer 2003, 44, 1979–1986. [Google Scholar] [CrossRef]
- Fan, C.F.; Hsu, S.L. Application of the molecular simulation technique to generate the structure of an aromatic polysulfone system. Macromolecules 1991, 24, 6244–6249. [Google Scholar] [CrossRef]
- Flory, F.H. Principles of Polymer Chemistry; Cornell University Press: Ithica, NY, USA, 1953; 672p. [Google Scholar]
- Guo, X.D.; Zhang, L.J.; Wu, Z.M.; Qian, Y. Dissipative particle dynamics studies on microstructure of pH-sensitive micelles for sustained drug delivery. Macromolecules 2010, 43, 7839–7844. [Google Scholar] [CrossRef]
- Connolly, M.L. Solvent-accessible surfaces of proteins and nucleic acids. Science 1983, 221, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.L. Computation of molecular volume. J. Am. Chem. Soc. 1985, 107, 1118–1124. [Google Scholar] [CrossRef]
- Bagchi, B.; Chakravarty, C. Interplay between multiple length and time scales in complex chemical systems. J. Chem. Sci. 2010, 122, 459–470. [Google Scholar] [CrossRef]
- Dai, X.; Shi, X.; Wang, Y.; Qiao, Y. Solubilization of salicylic acid in sapponin by ginsenoside Ro biosurfactant in aqueous solution: Mesoscopic simulation. J. Colloid Interface Sci. 2012, 384, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Soto-Figueroa, C.; Rodríguez-Hidalgo, M.; Vincente, L. Mesoscopic simulation of micellar-shuttle pathway of PB-PEO copolymer in a water/[BMIM][PF6] system. Chem. Phys. Lett. 2012, 531, 155–159. [Google Scholar] [CrossRef]
- Srinivas, G.; Cheng, X.; Smith, J.C. A solvent-free coarse grain model for crystalline and amorphous cellulose fibrils. J. Chem. Theory Comput. 2011, 7, 2539–2548. [Google Scholar] [CrossRef]







| pH | Solubility (mg/mL) | Ionized Salicylic Acid (%) |
|---|---|---|
| 2.00 | 15.3 ± 0.9 | 9.9 |
| 3.00 | 23.0 ± 1.3 | 51.7 |
| 4.00 | 30.6 ± 1.7 | 91.5 |
| 5.00 | >34.9 ± 3.2 | 99.1 |
| pH | kH (µg/cm2/h1/2) | R2 | % Swelling | Cumulative Release at 12 h (µg/cm2) | Donor pH t0 | Donor pH t12h |
|---|---|---|---|---|---|---|
| Unsaturated solutions | ||||||
| 2.00 | 237.0 ± 18 | 0.99 | 68.0 ± 3.5 | 180.7 ± 7.5 | 2.1 | 2.3 |
| 3.00 | 246.4 ± 33 | 0.99 | 71.0 ± 8.6 | 171.3 ± 7.4 | 3.0 | 2.9 |
| 4.00 | 197.5 ± 8.3 | 0.99 | 60.6 ± 1.6 | 234.3 ± 38 | 4.0 | 3.9 |
| 5.00 | 207.9 ± 22 | 0.99 | 63.1 ± 7.1 | 195.7 ± 8.4 | 5.0 | 4.9 |
| Saturated solutions | ||||||
| 2.00 | 6343.3 ± 658 | 1.0 | >140.4 ± 13 | 189.0 ± 4.0 | 2.1 | 2.6 |
| 3.00 | 6410.3 ± 359 | 1.0 | >96.4 ± 5.0 | 201.2 ± 12 | 3.0 | 3.1 |
| 4.00 | 48,587.3 ± 2240 | 1.0 | >569.9 ± 22 | 199.1 ± 8.5 | 4.1 | 3.6 |
| 5.00 | 89,877.5 ± 3233 | 0.99 | >919.3 ± 25 | 221.1 ± 3.5 | 5.1 | 4.2 |
| aij | Neutral | Charged | Propylene Glycol | Nitrocellulose | Water | Wall |
|---|---|---|---|---|---|---|
| Neutral | 25.00 a | |||||
| Charged | 2.000 b | 25.00 | ||||
| Propylene glycol | 32.64 | 2.000 | 25.00 | |||
| Nitrocellulose | 20.35 | 2.000 | 41.04 | 25.00 | ||
| Water | 37.49 | 2.000 | 26.16 | 56.90 | 25.00 | |
| Wall | 999 b | 999 | 999 | 999 | 999 | 25.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otto, D.P.; Combrinck, J.; Otto, A.; Tiedt, L.R.; De Villiers, M.M. Dissipative Particle Dynamics Investigation of the Transport of Salicylic Acid through a Simulated In Vitro Skin Permeation Model. Pharmaceuticals 2018, 11, 134. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040134
Otto DP, Combrinck J, Otto A, Tiedt LR, De Villiers MM. Dissipative Particle Dynamics Investigation of the Transport of Salicylic Acid through a Simulated In Vitro Skin Permeation Model. Pharmaceuticals. 2018; 11(4):134. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040134
Chicago/Turabian StyleOtto, Daniel P., Johann Combrinck, Anja Otto, Louwrens R. Tiedt, and Melgardt M. De Villiers. 2018. "Dissipative Particle Dynamics Investigation of the Transport of Salicylic Acid through a Simulated In Vitro Skin Permeation Model" Pharmaceuticals 11, no. 4: 134. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040134