Sucrosomial® Iron: A New Generation Iron for Improving Oral Supplementation
Abstract
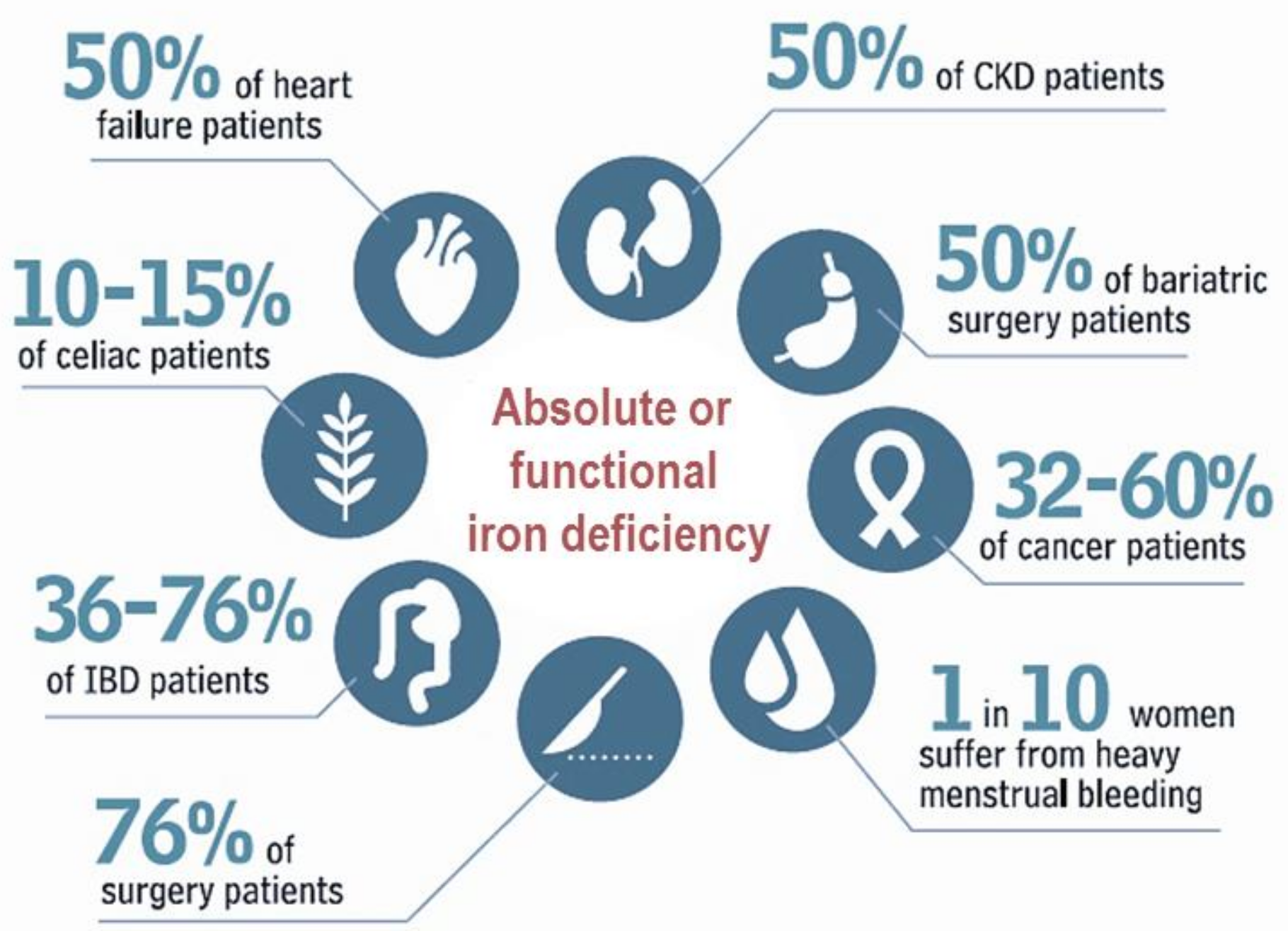
:1. Introduction
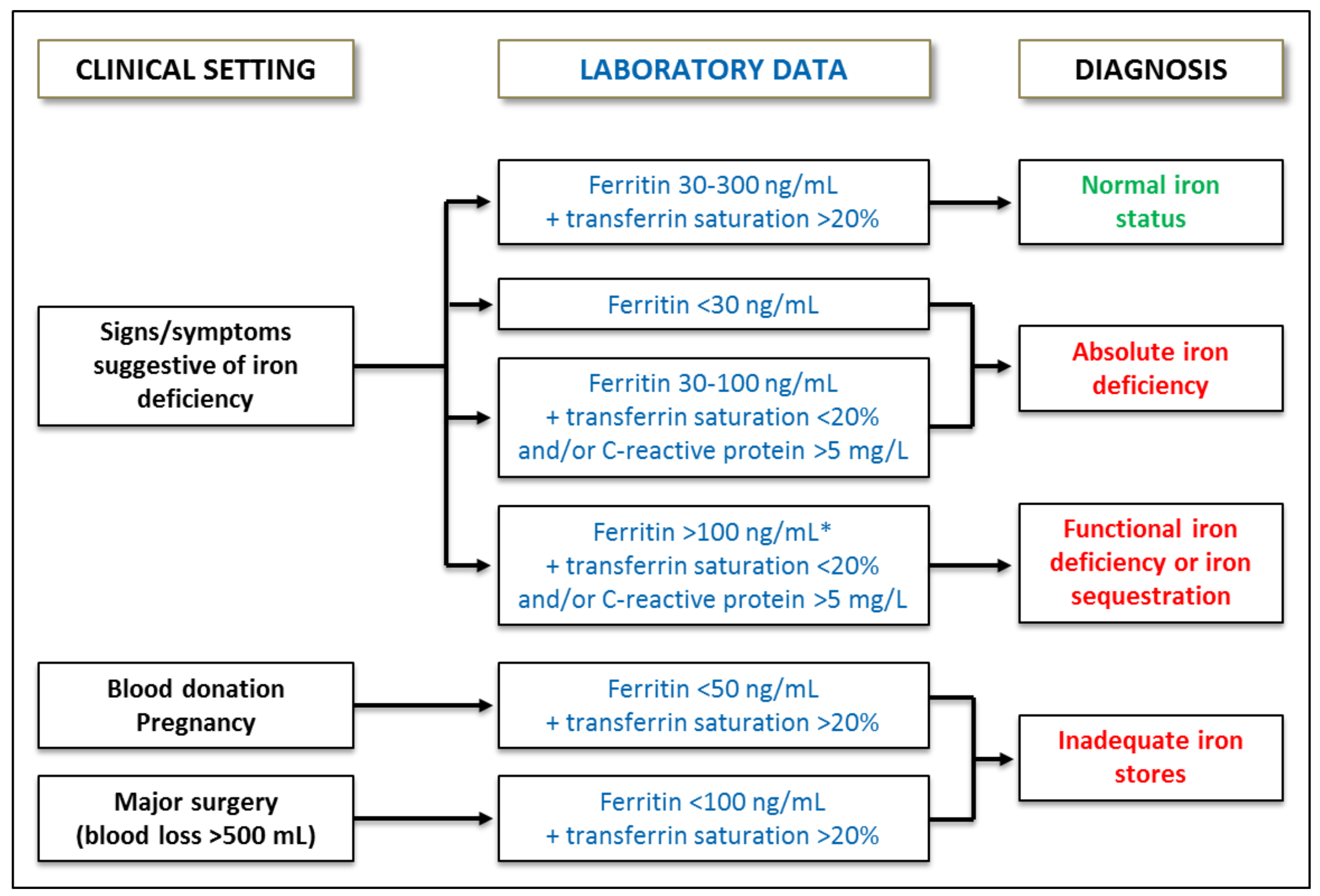
2. Diagnosis of Iron Deficiency
3. Treatment Options for Iron Deficiency
3.1. Oral Iron Supplementation
3.2. Intravenous Iron Supplementation
3.3. Red Blood Cell Transfusion
4. Sucrosomial® Iron: Preclinical Data
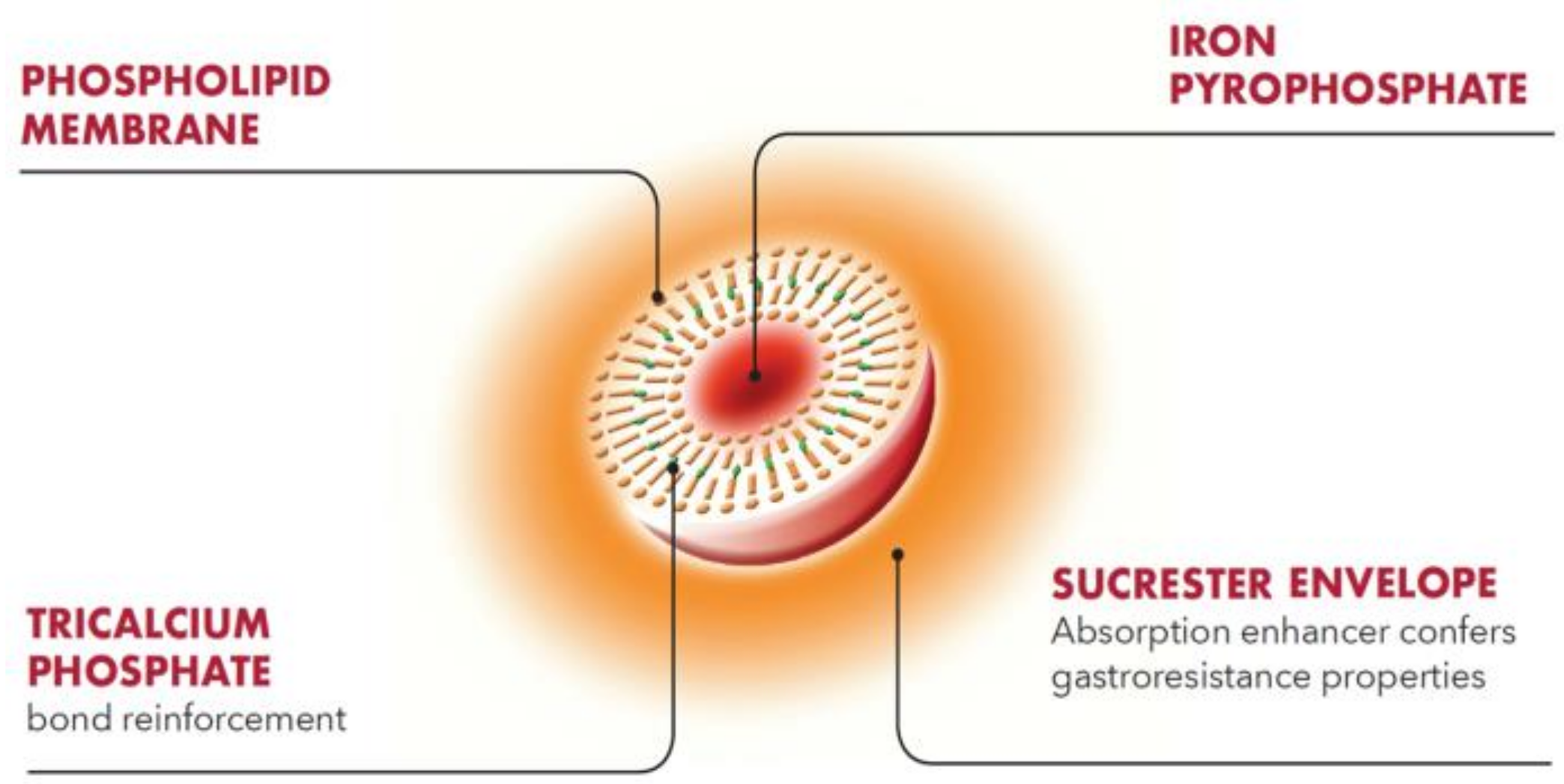
4.1. Composition and Structure
4.2. Gastro-Resistance and Intestinal Absorption
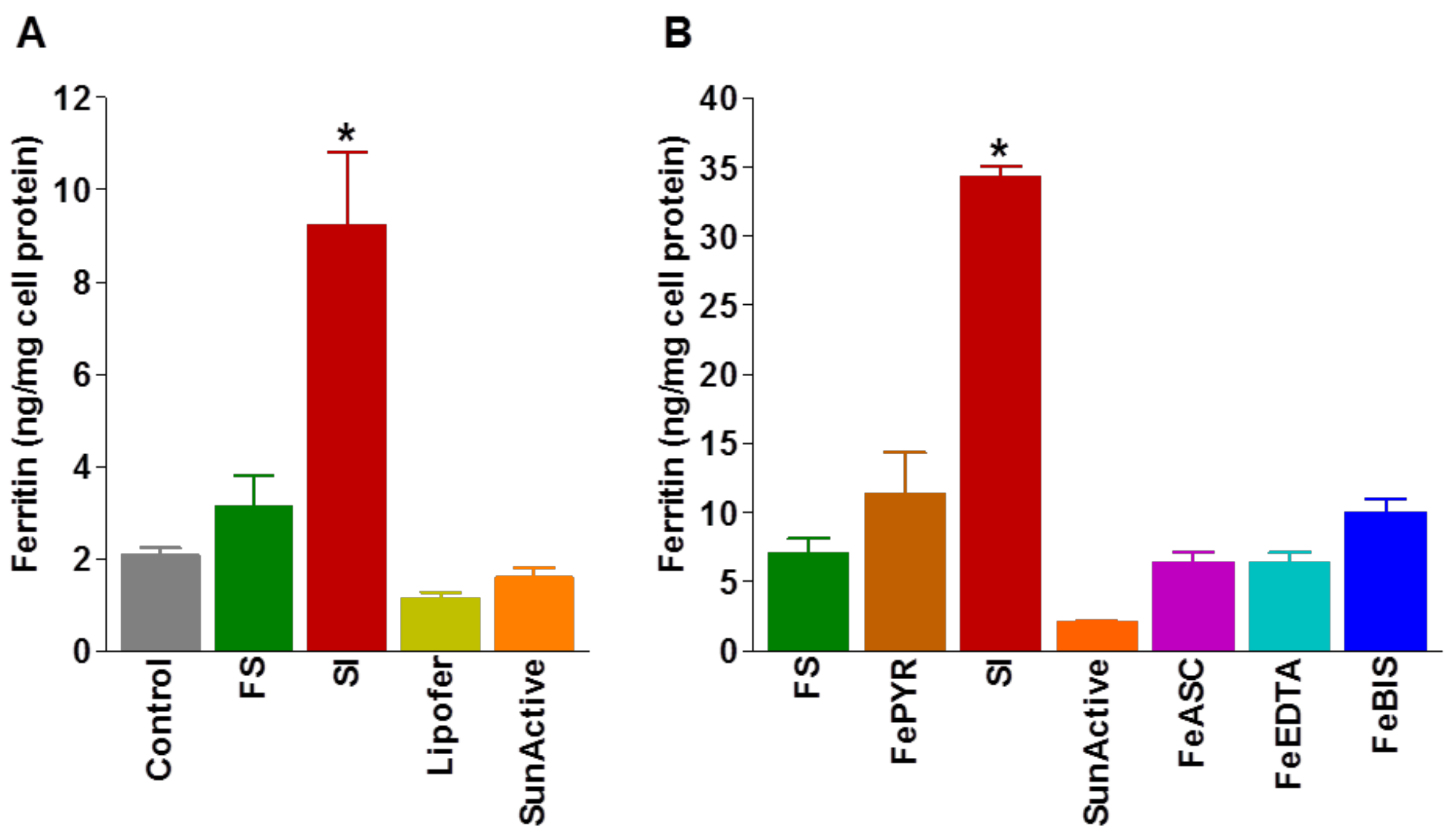
4.3. Bioavailability
4.4. Distribution
4.5. Iron Homeostasis
5. Sucrosomial® Iron for the Management of Iron Deficiency in Different Clinical Settings
5.1. Obstetrics
5.2. Oncology
5.3. Nephrology
5.4. Gastroenterology
5.5. Cardiology
5.6. Internal Medicine
5.7. Surgery
6. Efficacy of Sucrosomial® Iron: An Overview
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Remacha-Sevilla, Á.F.; Muñoz-Gómez, M. Anaemia in the elderly. Med. Clin. 2017, 149, 496–503. [Google Scholar] [CrossRef]
- Muñoz, M.; García-Erce, J.A.; Remacha, A.F. Disorders of iron metabolism. Part II: Iron deficiency and iron overload. J. Clin. Pathol. 2011, 64, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Peña-Rosas, J.P.; Robinson, S.; Milman, N.; Holzgreve, W.; Breymann, C.; Goffinet, F.; Nizard, J.; Christory, F.; Samama, C.M.; et al. Patient blood management in obstetrics: Management of anaemia and haematinic deficiencies in pregnancy and in the post-partum period: NATA consensus statement. Transfus. Med. 2018, 28, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascon, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Bircher, A.J.; Eckardt, K.U.; Obrador, G.T.; Pollock, C.A.; Stenvinkel, P.; Swinkels, D.W.; Wanner, C.; Weiss, G.; Chertow, G.M.; et al. Iron management in chronic kidney disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016, 89, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T.; Comin-Colet, J.; Leal-Noval, S.; Ozawa, S.; Takere, J.; Henry, D.; Javidroozi, M.; Hohmuth, B.; Bisbe, E.; Gross, I.; et al. Management of anemia in patients with congestive heart failure. Am. J. Hematol. 2017, 92, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegard, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohns Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Botella-Romero, F.; Gómez-Ramírez, S.; Campos, A.; Garcia-Erce, J.A. Iron deficiency and anaemia in bariatric surgical patients: Causes, diagnosis and proper management. Nutr. Hosp. 2009, 24, 640–654. [Google Scholar] [PubMed]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, M.; Gómez-Ramírez, S.; Campos, A.; Ruiz, J.; Liumbruno, G.M. Pre-operative anaemia: Prevalence, consequences and approaches to management. Blood Transfus. 2015, 13, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Laso-Morales, M.J.; Gómez-Ramírez, S.; Cladellas, M.; Nuñez-Matas, M.J.; Garcia-Erce, J.A. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia 2017, 72, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Adamson, J.W. How we diagnose and treat iron deficiency anemia. Am. J. Hematol. 2016, 91, 31–38. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L.; Iolascon, A.; Taher, A.; Cappellini, M.D. Clinical management of iron deficiency anemia in adults: Systemic review on advances in diagnosis and treatment. Eur. J. Intern. Med. 2017, 42, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Gómez-Ramírez, S.; Besser, M.; Pavia, J.; Gomollon, F.; Liumbruno, G.M.; Bhandari, S.; Cladellas, M.; Shander, A.; Auerbach, M. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus. 2017, 15, 422–437. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. WHO/NMH/NHD/MNM/11.1. Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 15 April 2018).
- Pratt, J.J.; Khan, K.S. Non-anaemic iron deficiency—A disease looking for recognition of diagnosis: A systematic review. Eur. J. Haematol. 2016, 96, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D. Diagnosis and management of iron-deficiency anaemia. Best Pract. Res. Clin. Haematol. 2005, 18, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Rao Baikady, R.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Walters, G.O.; Miller, F.M.; Worwood, M. Serum ferritin concentration and iron stores in normal subjects. J. Clin. Pathol. 1973, 26, 770–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barni, S.; Gascon, P.; Petrelli, F.; Garcia-Erce, J.A.; Pedrazzoli, P.; Rosti, G.; Giordano, G.; Mafodda, A.; Munoz, M. Position paper on management of iron deficiency in adult cancer patients. Expert Rev. Hematol. 2017, 10, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Gómez-Ramírez, S.; Bhandari, S. The safety of available treatment options for iron-deficiency anemia. Expert Opin. Drug Saf. 2018, 17, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.; Powell, J.J. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef] [PubMed]
- Cancelo-Hidalgo, M.J.; Castelo-Branco, C.; Palacios, S.; Haya-Palazuelos, J.; Ciria-Recasens, M.; Manasanch, J.; Perez-Edo, L. Tolerability of different oral iron supplements: A systematic review. Curr. Med. Res. Opin. 2013, 29, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: Two open-label, randomised controlled trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef]
- Rimon, E.; Kagansky, N.; Kagansky, M.; Mechnick, L.; Mashiah, T.; Namir, M.; Levy, S. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am. J. Med. 2005, 118, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. New Recommendations to Manage Risk of Allergic Reactions with Intravenous Iron Containing Medicines. EMA/579491/2013. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500151308.pdf (accessed on 18 July 2018).
- Leal-Noval, S.R.; Muñoz, M.; Asuero, M.; Contreras, E.; Garcia-Erce, J.A.; Llau, J.V.; Moral, V.; Paramo, J.A.; Quintana, M. Spanish Expert Panel on Alternatives to Allogeneic Blood T Spanish Consensus Statement on alternatives to allogeneic blood transfusion: The 2013 update of the “Seville Document”. Blood Transfus. 2013, 11, 585–610. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Marano, G.; Mengoli, C.; Pupella, S.; Vaglio, S.; Munoz, M.; Liumbruno, G.M. Red blood cell transfusion policy: A critical literature review. Blood Transfus. 2017, 15, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Guyatt, G.; Heddle, N.M.; Grossman, B.J.; Cohn, C.S.; Fung, M.K.; Gernsheimer, T.; Holcomb, J.B.; Kaplan, L.J.; Katz, L.M.; et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016, 316, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Vaglio, S.; Gentili, S.; Marano, G.; Pupella, S.; Rafanelli, D.; Biancofiore, G.; Antonioli, P.; Velati, C.; Liumbruno, G.M. The Italian Regulatory Guidelines for the implementation of Patient Blood Management. Blood Transfus. 2017, 15, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Ugolini, S.; Busti, F.; Marchi, G.; Castagna, A. Modern iron replacement therapy: Clinical and pathophysiological insights. Int. J. Hematol. 2018, 107, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Kis, L.; Szuts, A.; Otomo, N.; Szabo-Rvevz Deli, M.A. The Potential of sucrose esters to be used as oral absorption enhacers. Sci. Pharm. 2010, 78, 716. [Google Scholar] [CrossRef]
- Kis, L.; Hellinger, E.; Pilbat, A.M.; Kittel, A.; Torok, Z.; Furedi, A.; Szakacs, G.; Veszelka, S.; Sipos, P.; Ozsvari, B.; et al. Sucrose esters increase drug penetration, but do not inhibit p-glycoprotein in Caco-2 intestinal epithelial cells. J. Pharm. Sci. 2014, 103, 3107–3119. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, N.; Satsu, H.; Shimizu, M. Enhanced daunomycin accumulation in human intestinal Caco-2 cells from non-ionic food emulsifiers unrelated to the p-glycoprotein inhibitory mechanism. Biosci. Biotechnol. Biochem. 2006, 70, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Quintanar-Guerrero, D.; Ganem-Quintanar, A.; Allemann, E.; Fessi, H.; Doelker, E. Influence of the stabilizer coating layer on the purification and freeze-drying of poly(d,l-lactic acid) nanoparticles prepared by an emulsion-diffusion technique. J. Microencapsul. 1998, 15, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Brilli, E.; Fogli, S.; Beconcini, D.; Carpi, S.; Tarantino, G.; Zambito, Y. Sucrosomial® iron absorption studied by in vitro and ex-vivo models. Eur. J. Pharm. Sci. 2018, 111, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Brilli, E.; Romano, A.; Fabiano, A.; Zambito, Y.; Di Raimondo, F.; Tarantino, G. Sucrosomial® technology is able to promote ferric iron absorption: Pre-clinical and clinical evidences. Blood 2016, 128, 3618. [Google Scholar]
- Fabiano, A.; Brilli, E.; Mattii, L.; Testai, L.; Moscato, S.; Citi, V.; Tarantino, G.; Zambito, Y. Ex vivo and in vivo study of Sucrosomial® iron intestinal absorption and bioavailability. Int. J. Mol. Sci. 2018, 19, 2722. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Plapied, L.; Plaideau, C.; Legendre, D.; des Rieux, A.; Pourcelle, V.; Freichels, H.; Jerome, C.; Marchand, J.; Preat, V.; et al. In vitro identification of targeting ligands of human M cells by phage display. Int. J. Pharm. 2010, 394, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarantino, G.; Brilli, E.; Zambito, Y.; Giordano, G.; Equitani, F. Sucrosomial® iron: A new highly bioavailable oral iron supplement. Blood 2015, 126, 4561–4562. [Google Scholar]
- Starzynski, R.; Szudzik, M.; Staron, R.; Jonczy, A.; Smuda, E.; Pieszka, M.; Kamyczek, M.; Lipinski, P. Comparison of the therapeutical potential of oral Sucrosomial® iron and parenteral iron dextran supplementations in neonatal iron deficiency anemia in pigs. Am. J. Hematol. 2017, 92, E286. [Google Scholar]
- Asperti, A.; Gryzik, M.; Brilli, E.; Castagna, A.; Corbella, M.; Gottardo, R.; Girelli, D.; Tarantino, G.; Arosio, P.; Poli, M. Sucrosomial® iron supplementation in mice: Effects on blood parameters, hepcidin, and inflammation. Nutrients 2018, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Ferretti, F.; Branchi, F.; Tomba, C.; Lombardo, V.; Scricciolo, A.; Doneda, L.; Roncoroni, L. Sucrosomial® iron supplementation in anemic patients with celiac disease not tolerating oral ferrous sulfate: A prospective study. Nutrients 2018, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Ciudin, A.; Simo-Servat, O.; Balibrea, J.M.; Vilallonga, R.; Hernandez, C.; Simo, R.; Mesa, J. Response to oral Sucrosomial® iron supplementation in patients undergoing bariatric surgery. The BARI-FER study. Endocrinol. Diabetes Nutr. 2018, 65, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: A randomized trial. Nephrol. Dial. Transplant. 2015, 30, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Mafodda, A.; Giuffrida, D.; Prestifilippo, A.; Azzarello, D.; Giannicola, R.; Mare, M.; Maisano, R. Oral Sucrosomial® iron versus intravenous iron in anemic cancer patients without iron deficiency receiving darbepoetin alfa: A pilot study. Support. Care Cancer 2017, 25, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Capra, A.P.; Ferro, E.; Cannavò, L.; La Rosa, M.A.; Zirilli, G. A child with severe iron-deficiency anemia and a complex TMPRSS6 genotype. Hematology 2017, 22, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Brilli, E.; Barnadas, R.; Camacho, M.; Giordano, G.; Tarantino, G. Sucrosomial® Iron Absorption Involves M Cells Interaction. In Proceedings of the European Iron Club Annual Meeting, Zürich, Switzerland, 8–11 February 2018; p. 51. [Google Scholar]
- Rishi, G.; Subramaniam, V.N. The liver in regulation of iron homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G157–G165. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toblli, J.E.; Cao, G.; Olivieri, L.; Angerosa, M. Comparative study of gastrointestinal tract and liver toxicity of ferrous sulfate, iron amino chelate and iron polymaltose complex in normal rats. Pharmacology 2008, 82, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Nakanishi, H. Phosphatidylserine-containing liposomes: Potential pharmacological interventions against inflammatory and immune diseases through the production of prostaglandin E(2) after uptake by myeloid derived phagocytes. Arch. Immunol. Ther. Exp. 2011, 59, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Daru, J.; Zamora, J.; Fernandez-Felix, B.M.; Vogel, J.; Oladapo, O.T.; Morisaki, N.; Tuncalp, O.; Torloni, M.R.; Mittal, S.; Jayaratne, K.; et al. Risk of maternal mortality in women with severe anaemia during pregnancy and postpartum: A multilevel analysis. Lancet Glob. Health 2018, 6, e548–e554. [Google Scholar] [CrossRef]
- Fonseca, C.; Araujo, M.; Moniz, P.; Marques, F.; Araujo, I.; Costa, L.; Rodrigues, J.; Frade, L.; Botella, A.; Jesus, S.; et al. Prevalence and prognostic impact of anemia and iron deficiency in patients hospitalized in an internal medicine ward: The PRO-IRON study. Eur. J. Haematol. 2017, 99, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Berti, C.; Mando, C.; Martinelli, A.; Mazzali, C.; Cetin, I. Effects of different regimens of iron prophylaxis on maternal iron status and pregnancy outcome: A randomized control trial. J. Matern. Fetal Neonatal Med. 2017, 30, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Berardi, S.; Foltran, L.; Pascoli, I.; Pepe, A.; Salmeri, M.G.; Busato, E. Efficacy of oral Sucrosomial® iron in puerperium anemia. Exp. Rev. Hematol. 2016, 9 (Suppl. S1), 40. [Google Scholar] [CrossRef]
- Barni, S.; Lonati, V.; Ghilardi, M.; Borgonovo, K.F.; Cabiddu, M.; Astori, A.; Tarantino, G.; Petrelli, F. Upfront use of Sucrosomial® iron prevents transfusions in cancer patients on chemotherapy. Support. Care Cancer 2017, 25 (Suppl. S2), S144–S145. [Google Scholar]
- Locatelli, F.; Mazzaferro, S.; Yee, J. Iron Therapy Challenges for the Treatment of Nondialysis CKD Patients. Clin. J. Am. Soc. Nephrol. 2016, 11, 1269–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepshelovich, D.; Rozen-Zvi, B.; Avni, T.; Gafter, U.; Gafter-Gvili, A. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: An updated systematic review and meta-analysis. Am. J. Kidney Dis. 2016, 68, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Kusek, J.W.; Pappas, M.K. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015, 88, 905–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.U.; Gaillard, C.; Van Wyck, D.; Roubert, B.; Nolen, J.G.; Roger, S.D.; Investigators F-CS. FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol. Dial. Transplant. 2014, 29, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.U.; Gaillard, C.; Wyck, D.V.; Meier, Y.; Larroque, S.; Perrin, A.; Roger, S.D. Erythropoietic response to oral iron in patients with nondialysis-dependent chronic kidney disease in the FIND-CKD trial. Clin. Nephrol. 2017, 88, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Regidor, D.L.; McAllister, C.J.; Michael, B.; Warnock, D.G. Time-dependent associations between iron and mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Darba, J.; Ascanio, M. Budget Impact Analysis of Oral Fisiogen Ferro Forte((R)) versus Intravenous Iron for the Management of Iron Deficiency in Chronic Kidney Disease in Spain. Clin. Drug Investig. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Markopoulos, K.; Albertini, R.; Di Sabatino, A.; Biagi, F.; Ciccocioppo, R.; Arbustini, E.; Corazza, G.R. Anemia of chronic disease and defective erythropoietin production in patients with celiac disease. Haematologica 2008, 93, 1785–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.; Clavel, T.; Smirnov, K.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2017, 66, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Constante, M.; Fragoso, G.; Lupien-Meilleur, J.; Calve, A.; Santos, M.M. Iron Supplements Modulate Colon Microbiota Composition and Potentiate the Protective Effects of Probiotics in Dextran Sodium Sulfate-induced Colitis. Inflamm. Bowel Dis. 2017, 23, 753–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, J.; Aksan, A.; Farrag, K.; Dignass, A.; Radeke, H.H. Management of inflammatory bowel disease-related anemia and iron deficiency with specific reference to the role of intravenous iron in current practice. Expert Opin. Pharmacother. 2017, 18, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Bastida, G. Efficacy and tolerability of Sucrosomial iron supplementation in IBD patients with iron deficiency anemia and intolerance to iron oral salts. Exp. Rev. Hematol. 2016, 9 (Suppl. S1), 6–8. [Google Scholar] [CrossRef]
- Stuklov, N.I.; Basiladze, I.G.; Pivnik, A.V.; Knyazev, O.V.; Parfenov, A.I. Characteristics and modern treatment of iron deficiency syndromes in inflammatory bowel diseases. Exp. Rev. Hematol. 2018, 10 (Suppl. S1). in press. [Google Scholar]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676, quiz 677. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Camaschella, C. How I treat unexplained refractory iron deficiency anemia. Blood 2014, 123, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Farinati, F.; Maddalo, G. Iron and/or B12 deficient anemia in autoimmune gastritis. High dose sucrosomial iron supplementation: Preliminary data of a single center experience. Exp. Rev. Hematol. 2018, 10 (Suppl. S1). in press. [Google Scholar]
- Shipton, M.; Johal, N.; Dutta, N.; Ahmed, B.; Ammori, B.; Senapati, SP.; Akhtar, K.; Summers, L.; New, J.; Syed, A. Deficiencies of vitamin B12, folate and iron over 4 years of follow-up post-bariatric surgery [abstract]. Br. J. Surg. 2018, 105 (Suppl. S4), 28. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Malhotra, R.; Hernandez, A.F.; McNulty, S.E.; Smith, A.; Felker, G.M.; Tang, W.H.W.; LaRue, S.J.; Redfield, M.M.; Semigran, M.J.; et al. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial. JAMA 2017, 317, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Karavidas, A.; Trogkanis, E.; Farmakis, D.; Papingiotis, G.; Matzaraki, V.; Perpinia, A.; Parissis, J. Oral sucrosomial iron improves quality of life in heart failure patients with iron deficiency: A preliminary proof-of-concept study. Exp. Rev. Hematol. 2018, 10 (Suppl. S1). in press. [Google Scholar]
- Cabrera, P.; Gómez, S.; Herrero, V.; Martín, E.; Pavía, J.; Muñoz, M. Prevalence and consequences of anaemia among patients hospitalised at the internal medicine ward: A single centre audit. In Proceedings of the 15th European Congress of Internal Medicine, Amsterdam, The Netherlands, 2–3 September 2016. [Google Scholar]
- Fonseca, C.; Marques, F.; Robalo Nunes, A.; Belo, A.; Brilhante, D.; Cortez, J. Prevalence of anaemia and iron deficiency in Portugal: The EMPIRE study. Intern. Med. J. 2016, 46, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Mondello, P.; Tambaro, R.; Perrotta, N.; D’Amico, F.; D’Aveta, A.; Berardi, G.; Carabellese, B.; Patriarca, A.; Corbi, G.M.; et al. Biosimilar epoetin alpha is as effective as originator epoetin-alpha plus liposomal iron (Sideral(R)), vitamin B12 and folates in patients with refractory anemia: A retrospective real-life approach. Mol. Clin. Oncol. 2015, 3, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Santini, V. Clinical use of erythropoietic stimulating agents in myelodysplastic syndromes. Oncologist 2011, 16 (Suppl. S3), 35–42. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G. Reduction of inflammatory markers with liposomal iron (Sideral®). Pre-clinical and clinical results. Exp. Rev. Hematol. 2016, 9 (Suppl. S1), S17. [Google Scholar] [CrossRef]
- Giordano, G. Oral high-dose Sucrosomial® Iron vs. intravenous iron in sideropenic anemia intolerant/refractory to iron sulfate. Multicentric randomized study. Exp. Rev. Hematol. 2016, 9 (Suppl. S1), 15–17. [Google Scholar] [CrossRef]
- Giordano, G.; Parente, A.; Berardi, D.; Castaldi, D.; Cinotti, M.; Vedruccio, F.; Susca, V.; Petrella, L.; Berardi, G. Effectiveness of different oral iron formulations in iron deficiency anemia due to gastrointestinal bleeding: Multicentric randomized study; European Hematology Association: Stockholm, Sweden, 2018. [Google Scholar]
- Muñoz, M.; Franchini, M.; Liumbruno, G.M. The post-operative management of anaemia: More efforts are needed. Blood Transfus. 2018, 16, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Scardino, M.; Di Matteo, B.; Martorelli, F.; Tanzi, D.; Kon, E.; D’Amato, T. Improved patient blood management and cost saving in hip replacement surgery through the implementation of pre-operative Sucrosomial® iron supplementation: A quality improvement assessment study. Int. Orthop. 2018, 1–8, [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]

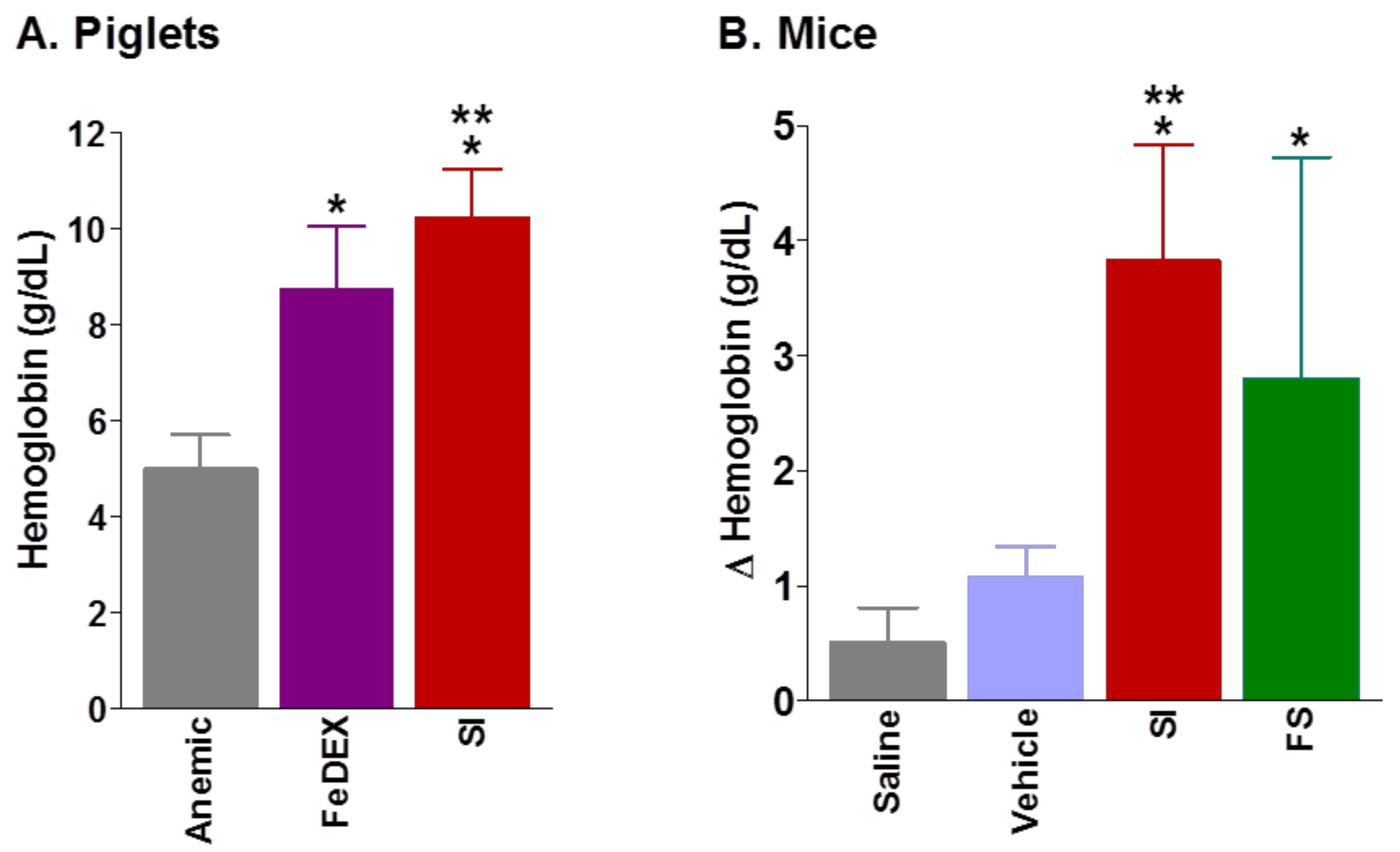
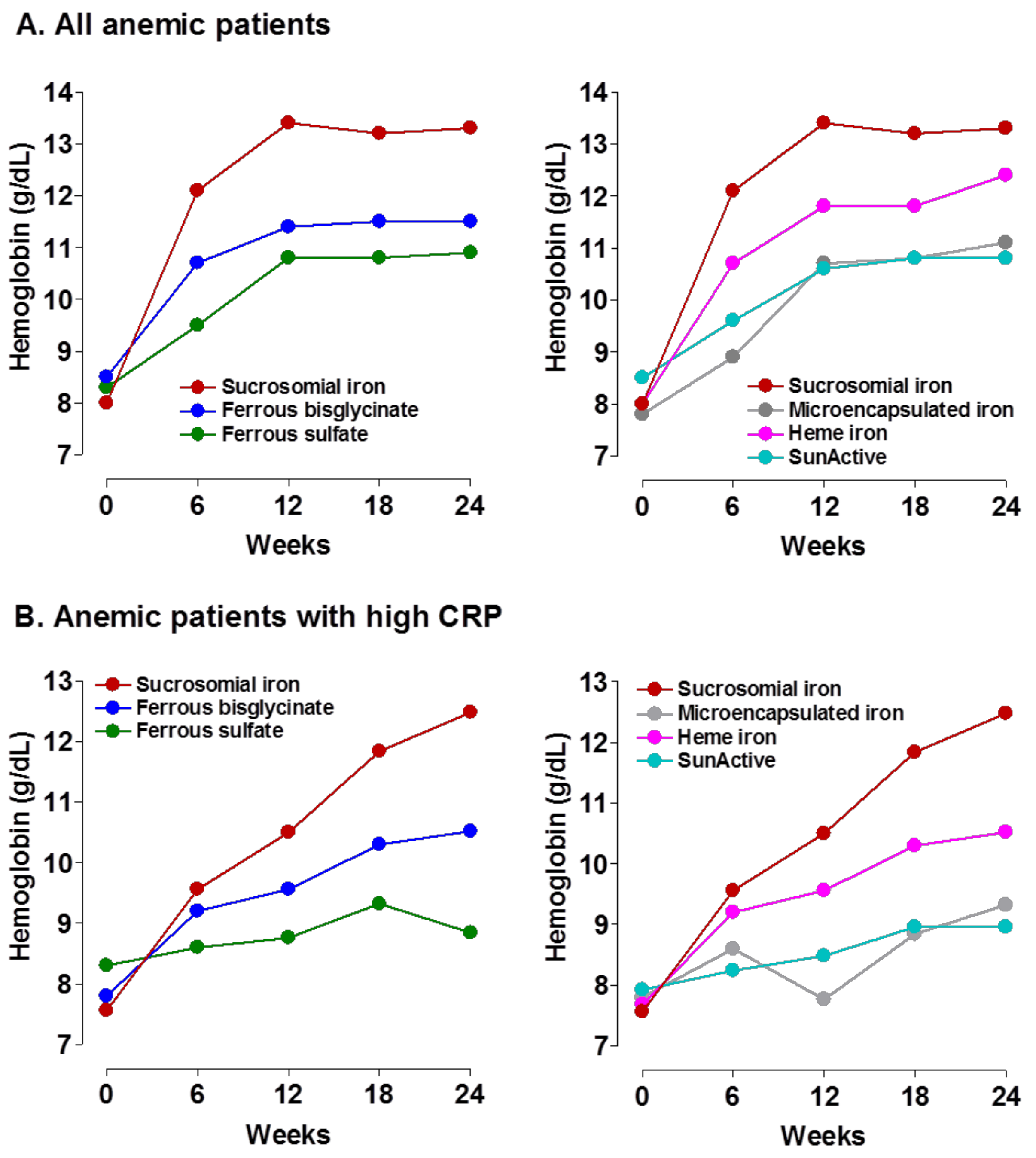
|
| Author [ref] (year) Study Type | Study Population | Treatment Compound (Dose) Duration | Baseline Hb (g/dL) | Final Hb (g/dL) | Baseline Ferritin (ng/mL) | Final Ferritin (ng/mL | Baseline TSAT (%) | Final TSAT (%) | GI Side Effects |
|---|---|---|---|---|---|---|---|---|---|
| Parisi et al. [60] (2017) RCT | 80 non-anemic pregnant women 12–14 week | Control, no iron (n = 20) FS (30 mg/day) (n = 20) SI (14 mg/day) (n = 20) SI (28 mg/day) (n = 20) Up to postpartum week 6 | 12.0 11.9 12.0 11.9 | 11.6 11.8 12.0 12.0 | 47 44 52 53 | 31 43 41 50 | 28 27 28 27 | 26 27 30 29 | |
| Mafodda et al. [51] (2017) RCT pilot | 64 patients with solid tumor | SI (30 mg/day) + DEPO (500 mcg/3 weeks) FG (125 mg/wk IV) + DEPO (500 mcg/3 weeks) 2 months | 9.4 9.2 | 12.7 12.9 | --- | --- | --- | --- | 3% 0% |
| Pisani et al. [50] (2014) RCT | 99 patients with chronic kidney disease | SI (30 mg/day) (n = 66) FG (125 mg/week IV, TID: 1000 mg) (n = 33) 3 months | 10.8 10.7 | 11.4 11.7 | 71 68 | 86 239 | 16.5 17.0 | 18.3 21.5 | 12% 18% |
| Bastida et al. [74] (2016) Case series | 46 patients with inflammatory bowel disease intolerant to FS | SI (30 mg/day) 3 months | 11.2 | 11.8 * | 14.3 | 16.0 | 8.7 | 16.2 | 11% |
| Stuklov et al. [75] (2018) Observational | 40 patients with inflammatory bowel disease | SI (60 mg/day) (n = 25) IS (100 mg/session, 500–1000 mg) (n = 15) 3 months | 10.1 10.0 | 11.8 11.8 | --- | --- | --- | --- | No |
| Elli et al. [48] (2016) Observational | 34 patients with celiac disease | SI (30 mg/day) intolerant to FS (n = 18) FS (105 mg/day) (n = 16) 3 months | 10.0 10.0 | 12.1 12.3 | 12 (all) | --- | 11 (all) | --- | Not stated |
| Farinati et al. [78] (2018) Case series | 20 patients with autoimmune atrophic gastritis | SI (120 mg/daily, either fasting or during meals) 8 weeks | 10.5 | 12.5 | 7 | 27 | 8 | 18 | 10% |
| Ciudín et al. [49] (2017) Case-control | 40 women after bariatric surgery | SI (28 mg/day) (n = 20) IVI (Iron sucrose 300 mg) (n = 20) 3 months | 12.4 12.5 | 12.3 12.7 | 102 98 | 89 96 | 22.9 23.6 | 24.1 26.3 | 0% 0% |
| Giordano et al. [87] (2016) RCT | 90 patient with IDA due to bleeding | SI (120 mg/day) (n = 45) FG (62.5 mg/day IV to cover TID) (n = 45) 4 weeks | 8.5 8.3 | 12.0 12.5 | 5 7 | --- | --- | --- | 26% 22% ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Ramírez, S.; Brilli, E.; Tarantino, G.; Muñoz, M. Sucrosomial® Iron: A New Generation Iron for Improving Oral Supplementation. Pharmaceuticals 2018, 11, 97. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040097
Gómez-Ramírez S, Brilli E, Tarantino G, Muñoz M. Sucrosomial® Iron: A New Generation Iron for Improving Oral Supplementation. Pharmaceuticals. 2018; 11(4):97. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040097
Chicago/Turabian StyleGómez-Ramírez, Susana, Elisa Brilli, Germano Tarantino, and Manuel Muñoz. 2018. "Sucrosomial® Iron: A New Generation Iron for Improving Oral Supplementation" Pharmaceuticals 11, no. 4: 97. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040097





