Analytical Characterization of an Inulin-Type Fructooligosaccharide from Root-Tubers of Asphodelusramosus L
Abstract
:1. Introduction
2. Results and Discussion
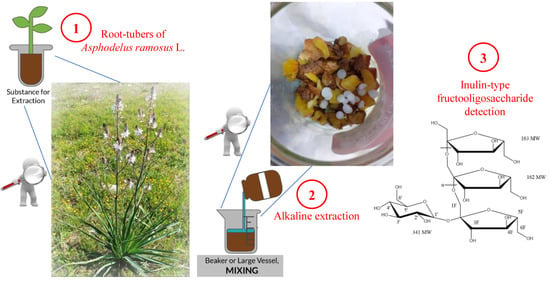
2.1. Extraction and Purification of Water-Soluble Polysaccharides from Root-Tubers of A. ramosus
2.2. High-Performance Thin-Layer Chromatography (HPTLC) Analysis
2.3. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.4. Nuclear Magnetic Resonance (NMR) Studies
3. Materials and Methods
3.1. Materials
3.2. Extraction and Purification of the Water-Soluble Polysaccharides from Root-Tubers of A. ramosus
3.3. High-Performance Thin-Layer Chromatography Analysis
3.4. FR-IR Analysis
3.5. NMR Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Seedi, H.R. Antimicrobial arylcoumarins from Asphodelus microcarpus. J. Nat. Prod. 2007, 70, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Malmir, M.; Serrano, R.; Caniça, M.; Silva-Lima, B.; Silva, O. A comprehensive review on the medicinal plants from the genus Asphodelus. Plants 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz Linfante, Z. Asphodelus L. In Flora Iberica, 1st ed.; Liliaceae-Agavaceae, Talavera, S., Andrés, C., Arista, M., Piedra, M.P.F., Rico, E., Crespo, M.B., Quintanar, A., Herrero, A., Aedo, C., Eds.; Real Jardin Botänico, Consejo Superior de Investigaciones Científicas (CSIC): Madrid, Spain, 2013; Volume 20, pp. 1–33. [Google Scholar]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional component of the Mediterranean diet. J. Ethnobiol. Ethnomed. 2018, 14, 14. [Google Scholar] [CrossRef] [Green Version]
- Peksel, A.; Imamoglu, S.; Altas Kiymaz, N.; Orhan, N. Antioxidant and radical scavenging activities of Asphodelus aestivus Brot. extracts. Int. J. Food Prop. 2013, 16, 1339–1350. [Google Scholar] [CrossRef]
- Crosti, R.; Ladd, P.G.; Dixon, K.W.; Piotto, B. Post-fire germination: The effect of smoke on seeds of selected species from the central Mediterranean basin. For. Ecol. Manag. 2006, 221, 306–312. [Google Scholar] [CrossRef]
- Perrino, E.V.; Signorile, G.; Marvulli, M. A first checklist of the vascular flora of the Polignano a Mare coast (Apulia, southern Italy). Nat. Croat. 2013, 22, 295–318. [Google Scholar]
- Adinolfi, M.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M.; Scopa, A.A. Bianthrone C glycoside from Asphodelus ramosus tubers. Phytochemistry 1989, 28, 284–288. [Google Scholar] [CrossRef]
- Adinolfi, M.; Lanzelta, R.; Marciano, C.E.; Parrilli, M.; Giulio, A.D.E. A new class of anthraquinone-anthrone-C-glycosides from Asphodelus ramosus tubers. Tetrahedron 1991, 47, 4435–4440. [Google Scholar] [CrossRef]
- Lanzetta, R.; Parrilli, M.; Adinolfi, M.; Aquila, T.; Corsaro, M.M. Bianthrone C-glycosides. 2. Three new compounds from Asphodelus ramosus tubers. Tetrahedron 1990, 46, 1287–1294. [Google Scholar] [CrossRef]
- Reynaud, J.; Lussignol, M.; Flament, M.M.; Becchi, M. Flavonoid content of Asphodelus ramosus (Liliaceae). Can. J. Bot. 1997, 75, 2105–2107. [Google Scholar] [CrossRef]
- Chimona, C.; Karioti, A.; Skaltsa, H.; Rhizopoulou, S. Occurrence of secondary metabolites in tepals of Asphodelus ramosus L. Plant Biosyst. 2014, 148, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Incoll, L.D.; Bonnett, G.D. The occurrence of fructan in food plants. In Studies in Plant Science, 1st ed.; Fuchs, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 3, pp. 309–322. [Google Scholar]
- Meier, H.; Reid, J.S.G. Reserve polysaccharides other than starch in higher plants. In Plant Carbohydrates I. Encyclopedia of Plant Physiology; New Series; Loewus, F.A., Tanner, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; Volume 13, pp. 418–471. [Google Scholar]
- Loewus, F.A.; Tanner, W. Plant Carbohydrates I: Intracellular Carbohydrates; New Series; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Franco-Robles, E.; López, M.G. Implication of fructans in health: Immunomodulatory and antioxidant mechanisms. Sci. World J. 2015, 2015, 289267. [Google Scholar]
- Brites, M.L.; Noreña, C.P.Z. Obtaining fructooligosaccharides from yacon (Smallanthus sonchifolius) by an ultrafiltration process. Braz. J. Chem. Eng. 2016, 33, 1011–1020. [Google Scholar] [CrossRef]
- Franck, A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 2002, 87, S287–S291. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef] [PubMed]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef]
- Ahmed, W.; Rashid, S. Functional and therapeutic potential of inulin: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–13. [Google Scholar] [CrossRef]
- Saeed, F.; Pasha, I.; Arshad, M.U.; Anjum, F.M.; Hussain, S.; Rasheed, R.; Nasir, M.A.; Shafique, B. Physiological and nutraceutical perspectives of fructan. Int. J. Food Prop. 2015, 18, 1895–1904. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Shang, H.M.; Zhou, H.Z.; Yang, J.Y.; Li, R.; Song, H.; Wu, H.X. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.L.; Zhang, M.; Zhou, H.L. Microwave-assisted extraction, purification, partial characterization, and bioactivity of polysaccharides from Panax ginseng. Molecules 2019, 24, 1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Lu, H.D.; Muḥammad, U.; Han, J.Z.; Wei, Z.H.; Lu, Z.X.; Bie, X.M.; Lu, F.X. Ultrasound-assisted extraction of polysaccharides from Artemisia selengensis Turcz and its antioxidant and anticancer activities. Int. J. Food Sci. 2016, 53, 1025–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wu, Q.; Yin, F.; Zhu, Z.; He, J.; Barba, F.J. Development of a combined trifluoroacetic acid hydrolysis and HPLC-ELSD method to identify and quantify inulin recovered from Jerusalem artichoke assisted by ultrasound extraction. Appl. Sci. 2018, 8, 710. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.Q.; Li, J.W.; Wang, Z.; Pan, H.X.; Chen, J.X.; Ning, Z.X. Optimization of alkaline extraction of polysaccharides from Ganoderma lucidum and their effect on immune function in mice. Molecules 2010, 15, 3694–3708. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yin, L.; Zhang, X.; Wang, Y.; Chen, Q.; Jin, C.; Hu, Y.; Wang, J. Optimization of alkaline extraction and bioactivities of polysaccharides from rhizome of Polygonatum odoratum. Biomed. Res. Int. 2014, 2014, 504896. [Google Scholar]
- Antal, M.J., Jr.; Mok, W.S.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose and sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Simonovska, B. Determination of inulin in foods. J. Assoc. Off. Anal. Chem. 2000, 83, 675–678. [Google Scholar] [CrossRef] [Green Version]
- Rizk, A.M.; Hammouda, F.M. Phytochemical Studies of Asphodelus microcarpus (Lipids and Carbohydrates). Planta Med. 1970, 18, 168–172. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, L.; Li, Y.; Cui, Y.; Jiang, S.; Tao, N.; Chen, H.; Zhao, Z.; Xu, J.; Dong, C. A novel inulin-type fructan from Asparagus cochinchinensis and its beneficial impact on human intestinal microbiota. Carbohydr. Polym. 2020, 247, 116761. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheong, K.; Song, Z.; Shi, Y.; Huang, X. Structure and protective effect on UVB-induced keratinocyte damage of fructan from white garlic. Carbohydr. Polym. 2013, 92, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xu, Y.; Chang, C.; Qiu, Z.; Hu, J.; Wu, Y.; Zhang, B.; Zheng, G. Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 2020, 163, 1677–1686. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, P.; Zhang, X.; Li, X. Chemical structure elucidation of an inulin-type fructan isolated from Lobelia chinensis lour with anti-obesity activity on diet-induced mice. Carbohydr. Polym. 2020, 240, 116357. [Google Scholar] [CrossRef]
- Cerantola, S.; Kervarec, N.; Pichon, R.; Magné, C.; Bessieres, M.A.; Deslandes, E. NMR characterization of inulin-type fructooligosaccharides as the major water-soluble carbohydrates from Matricaria maritima (L.). Carbohydr. Res. 2004, 339, 2445–2449. [Google Scholar] [CrossRef] [PubMed]
- Josephat, U.I.; Higginbotham, C.L. Polymer molecular weight analysis by 1H NMR spectroscopy. J. Chem. Educ. 2011, 88, 1098–1104. [Google Scholar]
- Ceccobelli, P.; Mirabella, C. WO-Composition of Asphodelus root extracts. PCT International Patent Application 2017/137887, 17 August 2017. [Google Scholar]



| Residue | H-1/C-1 (ppm) | H-2/C-2 (ppm) | H-3/C-3 (ppm) | H-4/C-4 (ppm) | H-5/C-5 (ppm) | H-6/C-6 (ppm) |
|---|---|---|---|---|---|---|
| →1)-β-d-Fruf-(2→ | 3.85/3.66 | - | 4.18 | 4.04 | 3.80 | 3.78/3.70 |
| 61.94 | 104.10 | 78.48 | 75.48 | 82.20 | 63.32 | |
| α-D-Glcp-(1→ | 5.35 | 3.49 | 3.69 | 3.48 | 3.88 | 3.75 |
| 93.38 | 72.26 | 73.49 | 70.31 | 72.66 | 61.42 |
Sample Availability: Samples of roots of Asphodelus ramosus L. are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madia, V.N.; De Vita, D.; Messore, A.; Toniolo, C.; Tudino, V.; De Leo, A.; Pindinello, I.; Ialongo, D.; Saccoliti, F.; D’Ursi, A.M.; et al. Analytical Characterization of an Inulin-Type Fructooligosaccharide from Root-Tubers of Asphodelusramosus L. Pharmaceuticals 2021, 14, 278. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14030278
Madia VN, De Vita D, Messore A, Toniolo C, Tudino V, De Leo A, Pindinello I, Ialongo D, Saccoliti F, D’Ursi AM, et al. Analytical Characterization of an Inulin-Type Fructooligosaccharide from Root-Tubers of Asphodelusramosus L. Pharmaceuticals. 2021; 14(3):278. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14030278
Chicago/Turabian StyleMadia, Valentina Noemi, Daniela De Vita, Antonella Messore, Chiara Toniolo, Valeria Tudino, Alessandro De Leo, Ivano Pindinello, Davide Ialongo, Francesco Saccoliti, Anna Maria D’Ursi, and et al. 2021. "Analytical Characterization of an Inulin-Type Fructooligosaccharide from Root-Tubers of Asphodelusramosus L" Pharmaceuticals 14, no. 3: 278. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14030278