A Comprehensive Review and Perspective on Natural Sources as Dipeptidyl Peptidase-4 Inhibitors for Management of Diabetes
Abstract
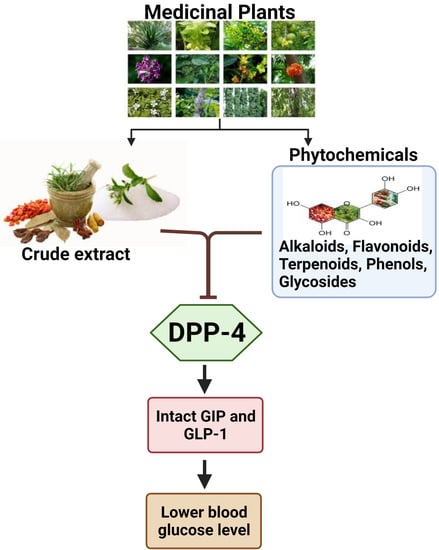
:1. Introduction
2. Dipeptidyl Peptidase-4
3. Incretin Hormones
4. Commercialized DPP-4 Inhibitors for the Treatment of Diabetes
5. DPP-4 Inhibition and Pancreatic Beta Cell Function
6. DPP-4 Inhibitors Improve Blood Glucose Response
7. DPP-4 Inhibition and Skeletal Muscle Function
8. DDP-4 Inhibitors from Natural Sources
8.1. Urena lobata
8.2. Anogeissus latifolia and Aegle marmelos
8.3. Castanospermum austral
8.4. Fagonia cretica and Hedera nepalensis
8.5. Eugenia jambolana and Pterocarpus marsupium
8.6. Chenopodium quinoa Willd
8.7. Allium sativum
8.8. Pilea microphylla
8.9. Mangifera indica
8.10. Lilium longiflorum
8.11. Coreopsis lanceolata
8.12. Psidium guajava L.
8.13. Melicope glabra
8.14. Hibiscus rosa-sinensis
8.15. Annona squamosa
8.16. Emblica officinalis
8.17. Berberis aristata
8.18. Avena sativa
8.19. Camellia sinensis
8.20. Vitis thunbergii var. taiwaniana
8.21. Prunus amygdalus
8.22. Ferula assa-foetida
8.23. Helichrysum arenarium
| S.No. | Plant Name | Family | Plant Part Used | Solvent/Extract Types | IC50 Value | Reference |
|---|---|---|---|---|---|---|
| 1. | Urena lobata | Malvaceae | Roots and leaves | Ethanol | 1.65 mg/mL | [76] |
| 2. | Castanospermum austral | Fabaceae | Seed | Ethanol | 13.96 µg/mL | [80] |
| 3. | Fagonia cretica | Zygophyllaceae | Aerial parts | Crude | 38.1 μg/mL | [81] |
| 4. | Hedera nepalensis | Araliaceae | Aerial parts | Crude | 17.2 μg/mL | [81] |
| 5. | Eugenia jambolana | Myrtaceae | Fruit | Fruit extract | 278.94 µg/mL | [82] |
| 6. | Pterocarpus marsupium | Leguminosae | Heartwood | Heartwood extract | 273.73 µg/mL | [82] |
| 7. | Chenopodium quinoa Willd | Amaranthaceae | Protein hydrolysates | - | 0.88 mg/mL | [83] |
| 8. | Allium sativum | Alliaceae | Garlic bulb | Methanol | 70.9 µg/mL | [85] |
| 9. | Pilea microphylla | Urticaceae | Leaves | Ethanol | 520.4 µg/mL | [86] |
| 10. | Mangifera indica | Anacardiaceae | Leaves | Methanol | 182.7 µg/mL | [88] |
| 11. | Psidium guajava | Myrtaceae | Leaves | Ethanol | 380 μg/mL | [92] |
| 12. | Melicope glabra | Rutaceae | Leaves | Chloroform | 169.40 μg/mL | [93] |
| 13. | Emblica officinalis | Phyllanthaceae | Fruit | Fruit extract | 3770 μg/mL | [97] |
| 14. | Berberis aristata | Berberidaceae | Bark | Methanol | 14.4 µg/mL | [98] |
| 15. | Camellia sinensis | Theaceae | Leaves | Ethanol | 227 µg/mL | [100] |
| 16. | Prunus amygdalus | Rosacease | Seed | Methanol | 162.9 µg/mL | [102] |
| 17. | Avena sativa | Poaceae | Seed | Seed flour | 0.99 mg/mL | [99] |
| 18. | Anogeissus latifolia | Combretaceae | Bark | Water | 754 µg/mL | [79] |
| 19. | Aegle marmelos | Rutaceae | Leaves | Water | 790 µg/mL | [79] |
| 20. | Helichrysum arenarium | Asteraceae | Flowers | Methanol | 41.2 µg/mL | [104] |
9. Natural Phytochemicals
9.1. Alkaloids
9.2. Flavonoids
9.3. Terpenoids
9.4. Phenols
9.5. Glycosides
| S.No. | Phytochemicals | IC50 Values | References |
|---|---|---|---|
| 1. | Alkaloids | ||
| Berberine | 13.3 µM | [106] | |
| 2. | Flavonoids | ||
| Cyanidin 3-O-glucoside | 125.1 µM | [108] | |
| Anthocyanins | 0.07 µM | [107] | |
| Emodin | 5.76 µM | [109] | |
| Rutin | 485 µM | [110] | |
| Isoquercetin | 96.8 µM | [114] | |
| Cirsimaritin | 0.43 µM | [112] | |
| Hispidulin | 0.49 µM | [112] | |
| Naringenin | 2.5 µM | [112] | |
| Quercetin | 4.02 nmol/mL | [113] | |
| Galangin | 40.13 µM | [69] | |
| Kaempferol 7-O-α-l-rhamnoside | 20.81 µM | [115] | |
| Vitexin | 33.12 µM | [115] | |
| Rutin | 32.93 µM | [115] | |
| 3. | Phenols | ||
| Hopeaphenol | 401 µM | [101] | |
| (+)-vitisin A | 90.75 µM | [101] | |
| (−)-vitisin B | 15.3 µM | [101] | |
| Resveratrol | 0.6 nM | [107] | |
| Luteolin | 0.12 µM | [107] | |
| Apigenin | 0.14 µM | [107] | |
| Flavone | 0.17 µM | [107] | |
| Coumarins | 54.83 nmol/mL | [113] | |
| Myricetin | 4.8 μM | [70] | |
| 4. | Glycosides | ||
| Kaempferol-3-O-β-gulcopyranosyl-(1→2)-β-galactopyranosyl-7-O-α-rhamnopyranoside | 27.89 μM | [121] | |
| 36.52 μM | |||
| Kaempferol-3-O-β-gulcopyranosyl-(1→2)-[α-rhamnopyranosyl (1→6)]-β-galactopyranosyl-7-O-α rhamnopyranoside | 37.01 μM | [121] | |
| Kaempferol-3-O-α-rhamnosyl (1→6)-O-β-galactopyranoside-7-O-α-rhamnopyranoside | 23.1 μM | [104] | |
| Chalconaringenin 2′-O-β-Dglucopyranoside | 24.3 μM | [104] | |
| Aureusidin 6-O-β-d-glucopyranoside | |||
| Quercetin-3-O-β-d-glucosyl-7-O-α-rhamnoside | 0.194 µg/mL | [122] | |
| Isorhamnetin-7-O-β-neohesperidoside | 0.573 µg/mL | [122] | |
| Isorhamnetin-3-O-β-d-glucoside | 0.345 µg/mL | [122] | |
| Kaempferol-40-methoxy-3,7-O-α-dirhamnoside | 0.281 µg/mL | [122] |
10. Bioactive Peptides
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Campbell, R.K. Fate of the beta-cell in the pathophysiology of type 2 diabetes. J. Am. Pharm. Assoc. 2009, 49, S10–S15. [Google Scholar] [CrossRef]
- Surampudi, P.N.; John-Kalarickal, J.; Fonseca, V.A. Emerging Concepts in the Pathophysiology of Type 2 Diabetes Mellitus. Mt. Sinai J. Med. A J. Transl. Pers. Med. 2009, 76, 216–226. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and Cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sozen, T.; Center, A.P.A.E.E.; Basaran, N.C.; Tinazli, M.; Ozisik, L. Musculoskeletal problems in diabetes mellitus. Eur. J. Rheumatol. 2018, 5, 258–265. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Al-Atram, A.A. A review of the bidirectional relationship between psychiatric disorders and diabetes mellitus. Neurosciences 2018, 23, 91–96. [Google Scholar] [CrossRef]
- Conarello, S.L.; Li, Z.; Ronan, J.; Roy, R.S.; Zhu, L.; Jiang, G.; Liu, F.; Woods, J.; Zycband, E.; Moller, D.E.; et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 6825–6830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marguet, D.; Baggio, L.; Kobayashi, T.; Bernard, A.-M.; Pierres, M.; Nielsen, P.F.; Ribel, U.; Watanabe, T.; Drucker, D.J.; Wagtmann, N. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc. Natl. Acad. Sci. USA 2000, 97, 6874–6879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianani, R. Beta cell regeneration in human pancreas. Semin. Immunopathol. 2010, 33, 23–27. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta Cell Dysfunction and Insulin Resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Saisho, Y. β-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J. Diabetes 2015, 6, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef] [Green Version]
- KAMESWARA, R.B.; Giri, R.; Kesavulu, M.; Apparao, C. Herbal medicine: In the management of diabetes mellitus. Manphar Vaidya Patrika 1997, 1, 33–35. [Google Scholar]
- Chang, C.L.T.; Lin, Y.; Bartolome, A.P.; Chen, Y.-C.; Chiu, S.-C.; Yang, W.-C. Herbal Therapies for Type 2 Diabetes Mellitus: Chemistry, Biology, and Potential Application of Selected Plants and Compounds. Evid. Based Complement. Altern. Med. 2013, 2013, 1–33. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Zhu, J.; Li, B.; Li, Z.; Zhu, W.; Shi, J.; Jia, Q.; Li, Y. Recent progress in natural products as DPP-4 inhibitors. Futur. Med. Chem. 2015, 7, 1079–1089. [Google Scholar] [CrossRef]
- Fukasawa, K.M.; Sahara, N.; Harada, M.; Kondo, Y.; Nagatsu, I. Immunohistochemical localization of dipeptidyl aminopeptidase IV in rat kidney, liver, and salivary glands. J. Histochem. Cytochem. 1981, 29, 337–343. [Google Scholar] [CrossRef]
- Matteucci, E.; Giampietro, O. Dipeptidyl peptidase-4 (CD26): Knowing the function before inhibiting the enzyme. Curr. Med. Chem. 2009, 16, 2943–2951. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, Y.R.; Yang, E.J.; Kwon, E.J.; Kim, S.H.; Kang, S.H.; Park, D.B.; Oh, B.C.; Kim, J.; Heo, S.T.; et al. CD26/DPP4 levels in peripheral blood and T cells in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2013, 98, 2553–2561. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juillerat-Jeanneret, L. Dipeptidyl peptidase IV and its inhibitors: Therapeutics for type 2 diabetes and what else? J. Med. Chem. 2014, 57, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Plosker, G.L. Sitagliptin: A review of its use in patients with type 2 diabetes mellitus. Drugs 2014, 74, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Vildagliptin: A review of its use in type 2 diabetes mellitus. Drugs 2014, 74, 587–610. [Google Scholar] [CrossRef]
- Dhillon, S. Saxagliptin: A Review in Type 2 Diabetes. Drugs 2015, 75, 1783–1796. [Google Scholar] [CrossRef]
- Keating, G.M. Alogliptin: A review of its use in patients with type 2 diabetes mellitus. Drugs 2015, 75, 777–796. [Google Scholar] [CrossRef]
- Lajara, R. Use of the dipeptidyl peptidase-4 inhibitor linagliptin in combination therapy for type 2 diabetes. Expert Opin. Pharmacother. 2012, 13, 2663–2671. [Google Scholar] [CrossRef]
- Nishio, S.; Abe, M.; Ito, H. Anagliptin in the treatment of type 2 diabetes: Safety, efficacy, and patient acceptability. Diabetes Metab. Syndr. Obes. 2015, 8, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Lee, S.H.; Yim, H.J. Gemigliptin, a novel dipeptidyl peptidase 4 inhibitor: First new anti-diabetic drug in the history of Korean pharmaceutical industry. Arch. Pharmacal Res. 2013, 36, 1185–1188. [Google Scholar] [CrossRef]
- Scott, L.J. Teneligliptin: A review in type 2 diabetes. Clin. Drug Investig. 2015, 35, 765–772. [Google Scholar] [CrossRef]
- McCormack, P.L. Evogliptin: First Global Approval. Drugs 2015, 75, 2045–2049. [Google Scholar] [CrossRef]
- Biftu, T.; Sinha-Roy, R.; Chen, P.; Qian, X.; Feng, D.; Kuethe, J.T.; Scapin, G.; Gao, Y.D.; Yan, Y.; Krueger, D.; et al. Omarigliptin (MK-3102): A novel long-acting DPP-4 inhibitor for once-weekly treatment of type 2 diabetes. J. Med. Chem. 2014, 57, 3205–3212. [Google Scholar] [CrossRef]
- Kaku, K. First novel once-weekly DPP-4 inhibitor, trelagliptin, for the treatment of type 2 diabetes mellitus. Expert Opin. Pharmacother. 2015, 16, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sun, H.; Piotrowski, D.W.; Ryder, T.F.; Doran, S.D.; Dai, H.; Prakash, C. Metabolism, excretion, and pharmacokinetics of ((3,3-difluoropyrrolidin-1-yl)((2S,4S)-4-(4-(pyrimidin-2-yl)piperazin-1-yl)pyrrol idin-2-yl)methanone, a dipeptidyl peptidase inhibitor, in rat, dog and human. Drug Metab. Dispos. 2012, 40, 2143–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deacon, C.F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: A comparative review. Diabetes Obes. Metab. 2011, 13, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.R.; DeYoung, M.B.; Lowe, C.; Parkes, D.G. GLP-1 receptor activated insulin secretion from pancreatic β-cells: Mechanism and glucose dependence. Diabetes Obes. Metab. 2013, 15, 15–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pospisilik, J.A.; Martin, J.; Doty, T.; Ehses, J.A.; Pamir, N.; Lynn, F.C.; Piteau, S.; Demuth, H.U.; McIntosh, C.H.; Pederson, R.A. Dipeptidyl peptidase IV inhibitor treatment stimulates β-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 2003, 52, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Ahren, B.; Foley, J.E. Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: Focus on alpha and beta cell function and lipid metabolism. Diabetologia 2016, 59, 907–917. [Google Scholar] [CrossRef]
- Argun-Kurum, G.; Kaya-Dagistanli, F.; Ozturk, M. DPP4 inhibitor induces beta cell regeneration and DDR-1 protein expression as an endocrine progenitor cell marker in neonatal STZ-diabetic rats. Pharmacol. Rep. 2019, 71, 721–731. [Google Scholar] [CrossRef]
- Omar, B.A.; Liehua, L.; Yamada, Y.; Seino, Y.; Marchetti, P.; Ahren, B. Dipeptidyl peptidase 4 (DPP-4) is expressed in mouse and human islets and its activity is decreased in human islets from individuals with type 2 diabetes. Diabetologia 2014, 57, 1876–1883. [Google Scholar] [CrossRef]
- Bugliani, M.; Syed, F.; Paula, F.M.M.; Omar, B.A.; Suleiman, M.; Mossuto, S.; Grano, F.; Cardarelli, F.; Boggi, U.; Vistoli, F.; et al. DPP-4 is expressed in human pancreatic beta cells and its direct inhibition improves beta cell function and survival in type 2 diabetes. Mol. Cell. Endocrinol. 2018, 473, 186–193. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Ohara-Imaizumi, M.; Nakamichi, Y.; Aoyagi, K.; Nishiwaki, C. DPP-4 inhibitor des-F-sitagliptin treatment increased insulin exocytosis from db/db mice beta cells. Biochem. Biophys. Res. Commun. 2011, 412, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.; Voelker, F.; Merriam, K.; Zhang, X.; Ren, X.; Subramanian, K.; Hughes, T.E.; Burkey, B.F. The DPP-4 inhibitor vildagliptin increases pancreatic beta cell mass in neonatal rats. Eur. J. Pharmacol. 2011, 650, 703–707. [Google Scholar] [CrossRef]
- Takeda, Y.; Fujita, Y.; Honjo, J.; Yanagimachi, T.; Sakagami, H.; Takiyama, Y.; Makino, Y.; Abiko, A.; Kieffer, T.J.; Haneda, M. Reduction of both beta cell death and alpha cell proliferation by dipeptidyl peptidase-4 inhibition in a streptozotocin-induced model of diabetes in mice. Diabetologia 2012, 55, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Han, S.J.; Choi, S.E.; Kang, Y.; Jung, J.G.; Yi, S.A.; Kim, H.J.; Lee, K.W.; Kim, D.J. Effect of sitagliptin plus metformin on beta-cell function, islet integrity and islet gene expression in Zucker diabetic fatty rats. Diabetes Res. Clin. Pract. 2011, 92, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Ardestani, A.; Dharmadhikari, G.; Laue, S.; Schumann, D.M.; Kerr-Conte, J.; Pattou, F.; Klein, T.; Maedler, K. The DPP-4 inhibitor linagliptin restores beta-cell function and survival in human isolated islets through GLP-1 stabilization. J. Clin. Endocrinol. Metab. 2013, 98, E1163–E1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akarte, A.S.; Srinivasan, B.P.; Gandhi, S. Vildagliptin selectively ameliorates GLP-1, GLUT4, SREBP-1c mRNA levels and stimulates beta-cell proliferation resulting in improved glucose homeostasis in rats with streptozotocin-induced diabetes. J. Diabetes Complicat. 2012, 26, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Suzuki, K.; Aoki, C.; Niitani, M.; Kato, K.; Tomotsune, T.; Aso, Y. Add-on treatment with teneligliptin ameliorates glucose fluctuations and improves glycemic control index in Japanese patients with type 2 diabetes on insulin therapy. Diabetes Technol. Ther. 2014, 16, 840–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, Y.; Taniguchi, Y.; Miyazaki, S.; Yokoyama, J.; Utsunomiya, K. Effects of add-on treatment with sitagliptin on narrowing the range of glucose fluctuations in Japanese type 2 diabetes patients receiving insulin therapy. Diabetes Technol. Ther. 2013, 15, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa-Tanaka, T.; Hosojima, M.; Kabasawa, H.; Kaseda, R.; Yasukawa, R.; Yata, Y.; Kuwahara, S.; Kono, E.; Takata, T.; Iino, N.; et al. Effects of DPP-4 Inhibitors on Blood Glucose Variability in Japanese Patients with Type 2 Diabetes on Maintenance Hemodialysis: A Prospective Observational Exploratory Study. Diabetes Ther. 2020, 11, 2845–2861. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Eiki, J.I.; Moritoyo, T.; Furihata, K.; Wakana, A.; Ohta, Y.; Tokita, S.; Kadowaki, T. Effect of short-term treatment with sitagliptin or glibenclamide on daily glucose fluctuation in drug-naive Japanese patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2018, 20, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Oh, S.; Jin, S.M.; Hur, K.Y.; Kim, J.H.; Lee, M.K. The efficacy and safety of adding either vildagliptin or glimepiride to ongoing metformin therapy in patients with type 2 diabetes mellitus. Expert Opin. Pharmacother. 2017, 18, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.H.; Hwang, Y.C.; Won, J.C.; Bae, J.C.; Kim, H.J.; Suh, S.; Lee, E.Y.; Lee, S.; Kim, S.Y.; Kim, J.H. Comparison of the effects of gemigliptin and dapagliflozin on glycaemic variability in type 2 diabetes: A randomized, open-label, active-controlled, 12-week study (STABLE II study). Diabetes Obes. Metab. 2020, 22, 173–181. [Google Scholar] [CrossRef]
- Cho, K.Y.; Nomoto, H.; Nakamura, A.; Kawata, S.; Sugawara, H.; Takeuchi, J.; Nagai, S.; Tsuchida, K.; Omori, K.; Yokoyama, H.; et al. Favourable effect of the sodium-glucose co-transporter-2 inhibitor canagliflozin plus the dipeptidyl peptidase-4 inhibitor teneligliptin in combination on glycaemic fluctuation: An open-label, prospective, randomized, parallel-group comparison trial (the CALMER study). Diabetes Obes. Metab. 2020, 22, 458–462. [Google Scholar] [CrossRef]
- Fuchigami, A.; Shigiyama, F.; Kitazawa, T.; Okada, Y.; Ichijo, T.; Higa, M.; Hiyoshi, T.; Inoue, I.; Iso, K.; Yoshii, H.; et al. Efficacy of dapagliflozin versus sitagliptin on cardiometabolic risk factors in Japanese patients with type 2 diabetes: A prospective, randomized study (DIVERSITY-CVR). Cardiovasc. Diabetol. 2020, 19, 1. [Google Scholar] [CrossRef] [Green Version]
- Lyu, F.P.; Huang, B.K.; Su, W.J.; Yan, F.F.; Zeng, J.Y.; Chen, Z.; Zhang, Y.X.; Wang, S.H.; Huang, Y.X.; Zhang, M.L.; et al. Efficacy of Vildagliptin Added to Continuous Subcutaneous Insulin Infusion (CSII) in Hospitalized Patients with Type 2 Diabetes. Diabetes Ther. 2020, 11, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Okajima, F.; Emoto, N.; Kato, K.; Sugihara, H. Effect of Glycemic Control on Chylomicron Metabolism and Correlation between Postprandial Metabolism of Plasma Glucose and Chylomicron in Patients with Type 2 Diabetes Treated with Basal-bolus Insulin Therapy with or without Vildagliptin. J. Atheroscler. Thromb. 2017, 24, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Ahren, B.; Foley, J.E.; Ferrannini, E.; Matthews, D.R.; Zinman, B.; Dejager, S.; Fonseca, V.A. Changes in prandial glucagon levels after a 2-year treatment with vildagliptin or glimepiride in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care 2010, 33, 730–732. [Google Scholar] [CrossRef] [Green Version]
- Ahren, B.; Schweizer, A.; Dejager, S.; Dunning, B.E.; Nilsson, P.M.; Persson, M.; Foley, J.E. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 1236–1243. [Google Scholar] [CrossRef] [Green Version]
- Li, F.F.; Shen, Y.; Sun, R.; Zhang, D.F.; Jin, X.; Zhai, X.F.; Chen, M.Y.; Su, X.F.; Wu, J.D.; Ye, L.; et al. Effects of Vildagliptin Add-on Insulin Therapy on Nocturnal Glycemic Variations in Uncontrolled Type 2 Diabetes. Diabetes Ther. 2017, 8, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.; Schweizer, A.; Albrecht, D.; Baron, M.A.; Chang, I.; Dejager, S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 2007, 50, 1148–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.C.; Dettbarn, W.-D.; Milatovic, D. Skeletal Muscle. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier: Amsterdam, The Netherlands, 2009; pp. 509–531. [Google Scholar]
- Kluess, H.A. Dipeptidyl Peptidase IV as a Muscle Myokine. Front. Physiol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Raschke, S.; Eckardt, K.; Bjorklund Holven, K.; Jensen, J.; Eckel, J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS ONE 2013, 8, e62008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neidert, L.E.; Al-Tarhuni, M.; Goldman, D.; Kluess, H.A.; Jackson, D.N. Endogenous dipeptidyl peptidase IV modulates skeletal muscle arteriolar diameter in rats. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Bouchi, R.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Dipeptidyl peptidase 4 inhibitors attenuates the decline of skeletal muscle mass in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2018, 34. [Google Scholar] [CrossRef]
- Ishii, S.; Nagai, Y.; Kato, H.; Fukuda, H.; Tanaka, Y. Effect of the Dipeptidyl Peptidase-4 Inhibitor Sitagliptin on Muscle Mass and the Muscle/Fat Ratio in Patients With Type 2 Diabetes. J. Clin. Med. Res. 2020, 12, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Kalhotra, P.; Chittepu, V.C.S.R.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Chrysin in Combination with Insulin Promotes Glucose Uptake in Skeletal Muscle Cell: Impact of Combination Therapy in Diabetes Myopathy (P01-031-19). Curr. Dev. Nutr. 2019, 3, nzz028-P01. [Google Scholar] [CrossRef] [Green Version]
- Kalhotra, P.; Chittepu, V.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Discovery of Galangin as a Potential DPP-4 Inhibitor That Improves Insulin-Stimulated Skeletal Muscle Glucose Uptake: A Combinational Therapy for Diabetes. Int. J. Mol. Sci. 2019, 20, 1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalitha, N.; Sadashivaiah, B.; Ramaprasad, T.R.; Singh, S.A. Anti-hyperglycemic activity of myricetin, through inhibition of DPP-4 and enhanced GLP-1 levels, is attenuated by co-ingestion with lectin-rich protein. PLoS ONE 2020, 15, e0231543. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Shaikh, S.; Ahmad, K.; Ahmad, S.S.; Lim, J.H.; Park, S.; Yang, H.J.; Cho, W.K.; Park, S.J.; Lee, Y.H.; et al. Isolation and Characterization of Compounds from Glycyrrhiza uralensis as Therapeutic Agents for the Muscle Disorders. Int. J. Mol. Sci. 2021, 22, 876. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.J.R.; Quave, C.L.; Collemare, J.; Tatsis, E.C.; Twilley, D.; Lulekal, E.; Farlow, A.; Li, L.; Cazar, M.E.; Leaman, D.J. Molecules from nature: Reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet 2020, 2, 463–481. [Google Scholar] [CrossRef]
- Baig, M.H.; Jan, A.T.; Rabbani, G.; Ahmad, K.; Ashraf, J.M.; Kim, T.; Min, H.S.; Lee, Y.H.; Cho, W.K.; Ma, J.Y.; et al. Methylglyoxal and Advanced Glycation End products: Insight of the regulatory machinery affecting the myogenic program and of its modulation by natural compounds. Sci. Rep. 2017, 7, 5916. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Onoagbe, I.; Negbenebor, E.; Ogbeide, V.; Dawha, I.; Attah, V.; Lau, H.; Omonkhua, A. A study of the anti-diabetic effects of Urena lobata and Sphenostylis stenocarpa in streptozotocin-induced diabetic rats. Eur. J. Sci. Res. 2010, 43, 6–14. [Google Scholar]
- Purnomo, Y.; Soeatmadji, D.W.; Sumitro, S.B.; Widodo, M.A. Anti-diabetic potential of Urena lobata leaf extract through inhibition of dipeptidyl peptidase IV activity. Asian Pac. J. Trop. Biomed. 2015, 5, 645–649. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, S.; Faisal, T.K.; Anjumary, J.; Rajasekaran, A.; Asokkumar, K.; Annadurai, K.; Arivukkarasu, R.; Sharma, R.K.; Shankar, M.B. Comparative evaluation of hypoglycemic and hypolipidemic activity of various extract of Anogeissus latifolia bark in streptozotocin-induced diabetic rats. J. Complement. Integr. Med. 2017, 14. [Google Scholar] [CrossRef]
- Baliga, M.S.; Thilakchand, K.R.; Rai, M.P.; Rao, S.; Venkatesh, P. Aegle marmelos (L.) Correa (Bael) and its phytochemicals in the treatment and prevention of cancer. Integr. Cancer Ther. 2013, 12, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, P.; Hannon-Fletcher, M.P.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Effects of 22 traditional anti-diabetic medicinal plants on DPP-IV enzyme activity and glucose homeostasis in high-fat fed obese diabetic rats. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Krishnan, S.; Kumar, A.; Rajak, K.K.; Murari, K.; Bharti, B.K.; Gupta, A.K. Antihyperglycemic activity with DPP-IV inhibition of alkaloids from seed extract of Castanospermum australe: Investigation by experimental validation and molecular docking. Phytomedicine 2012, 20, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Jafri, L.; Haq, I.U.; Chang, L.C.; Calderwood, D.; Green, B.D.; Mirza, B. Plants Fagonia cretica L. and Hedera nepalensis K. Koch contain natural compounds with potent dipeptidyl peptidase-4 (DPP-4) inhibitory activity. J. Ethnopharmacol. 2014, 156, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, J.; Dubala, A.; Chinni, S.; Khatwal, R.B.; Satish Kumar, M.N.; Basavan, D. A molecular connection of Pterocarpus marsupium, Eugenia jambolana and Gymnema sylvestre with dipeptidyl peptidase-4 in the treatment of diabetes. Pharm. Biol. 2014, 52, 268–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nongonierma, A.B.; Le Maux, S.; Dubrulle, C.; Barre, C.; FitzGerald, R.J. Quinoa (Chenopodium quinoa Willd.) protein hydrolysates with in vitro dipeptidyl peptidase IV (DPP-IV) inhibitory and antioxidant properties. J. Cereal Sci. 2015, 65, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [PubMed]
- Kalhotra, P.; Chittepu, V.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Phytochemicals in Garlic Extract Inhibit Therapeutic Enzyme DPP-4 and Induce Skeletal Muscle Cell Proliferation: A Possible Mechanism of Action to Benefit the Treatment of Diabetes Mellitus. Biomolecules 2020, 10, 305. [Google Scholar] [CrossRef] [Green Version]
- Bansal, P.; Paul, P.; Mudgal, J.; Nayak, P.G.; Pannakal, S.T.; Priyadarsini, K.I.; Unnikrishnan, M.K. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp. Toxicol. Pathol. 2012, 64, 651–658. [Google Scholar] [CrossRef]
- Aderibigbe, A.O.; Emudianughe, T.S.; Lawal, B.A. Evaluation of the antidiabetic action of Mangifera indica in mice. Phytother. Res. 2001, 15, 456–458. [Google Scholar] [CrossRef]
- Yogisha, S.; Raveesha, K.A. Dipeptidyl Peptidase IV inhibitory activity of Mangifera indica. J. Nat. Prod. 2010, 3, 9. [Google Scholar]
- Suman, R.K.; Mohanty, I.R.; Maheshwari, U.; Borde, M.K.; Deshmukh, Y.A. Natural dipeptidyl peptidase-IV inhibitor mangiferin mitigates diabetes- and metabolic syndrome-induced changes in experimental rats. Diabetes Metab. Syndr. Obes. 2016, 9, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.R.; Thapa, P.; Kim, H.M.; Jin, C.H.; Kim, S.H.; Kim, J.B.; Choi, H.; Han, A.R.; Nam, J.W. Purification of Phenylpropanoids from the Scaly Bulbs of Lilium Longiflorum by CPC and Determination of Their DPP-IV Inhibitory Potentials. ACS Omega 2020, 5, 4050–4057. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.R.; Paudel, S.B.; Nam, J.W.; Jin, C.H.; Lee, I.S.; Han, A.R. Constituents of Coreopsis lanceolata Flower and Their Dipeptidyl Peptidase IV Inhibitory Effects. Molecules 2020, 25, 4370. [Google Scholar] [CrossRef]
- Eidenberger, T.; Selg, M.; Krennhuber, K. Inhibition of dipeptidyl peptidase activity by flavonol glycosides of guava (Psidium guajava L.): A key to the beneficial effects of guava in type II diabetes mellitus. Fitoterapia 2013, 89, 74–79. [Google Scholar] [CrossRef]
- Quek, A.; Kassim, N.K.; Ismail, A.; Latif, M.A.M.; Shaari, K.; Tan, D.C.; Lim, P.C. Identification of Dipeptidyl Peptidase-4 and alpha-Amylase Inhibitors from Melicope glabra (Blume) T. G. Hartley (Rutaceae) Using Liquid Chromatography Tandem Mass Spectrometry, In Vitro and In Silico Methods. Molecules 2020, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Azam, S.; Hannan, J.M.A.; Flatt, P.R.; Abdel Wahab, Y.H.A. Anti-hyperglycaemic activity of H. rosa-sinensis leaves is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of insulin secretion. J. Ethnopharmacol. 2020, 253, 112647. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Evaluation of the Antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants 2020, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Majeed, S.; Mundkur, L.; Nagabhushanam, K.; Arumugam, S.; Beede, K.; Ali, F. Standardized Emblica officinalis fruit extract inhibited the activities of alpha-amylase, alpha-glucosidase, and dipeptidyl peptidase-4 and displayed antioxidant potential. J. Sci. Food Agric. 2020, 100, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, R.; Bhavtaran, S.; Narendra, P.; Varghese, N.; Vanchhawng, L.; Mohamed Sham Shihabudeen, H.; Thirumurgan, K. Dipeptidyl peptidase-IV inhibitory activity of Berberis aristata. J. Nat. Prod. 2011, 4, 158–163. [Google Scholar]
- Wang, F.; Yu, G.; Zhang, Y.; Zhang, B.; Fan, J. Dipeptidyl Peptidase IV Inhibitory Peptides Derived from Oat (Avena sativa L.), Buckwheat (Fagopyrum esculentum), and Highland Barley (Hordeum vulgare trifurcatum (L.) Trofim) Proteins. J. Agric. Food Chem. 2015, 63, 9543–9549. [Google Scholar] [CrossRef]
- Ekayanti, M.; Sauriasari, R.; Elya, B. Dipeptidyl peptidase IV inhibitory activity of fraction from white tea ethanolic extract (Camellia sinensis (L.) Kuntze) ex vivo. Pharmacogn. J. 2018, 10, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.S.; Chen, C.R.; Wu, W.H.; Wen, C.L.; Chang, C.I.; Hou, W.C. Anti-alpha-glucosidase and Anti-dipeptidyl Peptidase-IV Activities of Extracts and Purified Compounds from Vitis thunbergii var. taiwaniana. J. Agric. Food Chem. 2015, 63, 6393–6401. [Google Scholar] [CrossRef]
- Kumar, V.; Sachan, R.; Rahman, M.; Sharma, K.; Al-Abbasi, F.A.; Anwar, F. Prunus amygdalus extract exert antidiabetic effect via inhibition of DPP-IV: In-silico and in-vivo approaches. J. Biomol. Struct. Dyn. 2020, 1–15. [Google Scholar] [CrossRef]
- YARIZADE, A.; KUMLEH, H.H.; Niazi, A. In vitro antidiabetic effects of ferula assa-foetida extracts through dipeptidyl peptidase iv and α-glucosidase inhibitory activity. IN VITRO 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, T.; Ninomiya, K.; Akaki, J.; Kakihara, N.; Kuramoto, H.; Matsumoto, Y.; Hayakawa, T.; Muraoka, O.; Wang, L.B.; Wu, L.J.; et al. Dipeptidyl peptidase-IV inhibitory activity of dimeric dihydrochalcone glycosides from flowers of Helichrysum arenarium. J. Nat. Med. 2015, 69, 494–506. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-masri, I.M.; Mohammad, M.K.; Tahaa, M.O. Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J. Enzym. Inhib. Med. Chem. 2009, 24, 1061–1066. [Google Scholar] [CrossRef]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; de Mejia, E.G. Berry and Citrus Phenolic Compounds Inhibit Dipeptidyl Peptidase IV: Implications in Diabetes Management. Evid. Based Complement. Altern. Med. 2013, 2013, 479505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cásedas, G.; Les, F.; González-Burgos, E.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Cyanidin-3-O-glucoside inhibits different enzymes involved in central nervous system pathologies and type-2 diabetes. S. Afr. J. Bot. 2019, 120, 241–246. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Fan, H.; Wu, P.; Zhang, F.; Zhang, C.; Liu, W.; Li, M. Screening of a natural compound library identifies emodin, a natural compound from Rheum palmatum Linn that inhibits DPP4. PeerJ 2017, 5, e3283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Jacobson, G.A.; Burgess, J.R.; Jelinek, H.F.; Nichols, D.S.; Narkowicz, C.K.; Al-Aubaidy, H.A. Citrus bioflavonoids dipeptidyl peptidase-4 inhibition compared with gliptin antidiabetic medications. Biochem. Biophys. Res. Commun. 2018, 503, 21–25. [Google Scholar] [CrossRef]
- Parmar, H.S.; Jain, P.; Chauhan, D.S.; Bhinchar, M.K.; Munjal, V.; Yusuf, M.; Choube, K.; Tawani, A.; Tiwari, V.; Manivannan, E.; et al. DPP-IV inhibitory potential of naringin: An in silico, in vitro and in vivo study. Diabetes Res. Clin. Pract. 2012, 97, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; de Mejia, E.G. Bioactive compounds from culinary herbs inhibit a molecular target for type 2 diabetes management, dipeptidyl peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Patel, P.K.; Choudhary, K.; Joshi, J.; Yadav, D.; Jin, J.O. Quercetin and Coumarin Inhibit Dipeptidyl Peptidase-IV and Exhibits Antioxidant Properties: In Silico, In Vitro, Ex Vivo. Biomolecules 2020, 10, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, S.-T.; Yin, Y.-C.; Xing, S.; Li, W.-N.; Fu, X.-Q. Hypoglycemic effect and mechanism of isoquercitrin as an inhibitor of dipeptidyl peptidase-4 in type 2 diabetic mice. RSC Adv. 2018, 8, 14967–14974. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.T.; Le, D.D.; Nguyen, P.H.; Ali, M.Y.; Choi, J.S.; Min, B.S.; Shin, H.M.; Rhee, H.I.; Woo, M.H. PTP1B, alpha-glucosidase, and DPP-IV inhibitory effects for chromene derivatives from the leaves of Smilax china L. Chem. Biol. Interact. 2016, 253, 27–37. [Google Scholar] [CrossRef]
- Katkar, K.V.; Suthar, A.C.; Chauhan, V.S. The chemistry, pharmacologic, and therapeutic applications of Polyalthia longifolia. Pharmacogn. Rev. 2010, 4, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.K.; Lin, S.R.; Riyaphan, J.; Fu, Y.S.; Weng, C.F. Polyalthia Clerodane Diterpene Potentiates Hypoglycemia via Inhibition of Dipeptidyl Peptidase 4. Int. J. Mol. Sci. 2019, 20, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Y.; Lu, Z.M.; Huang, W.; Xu, H.Y.; Shi, J.S.; Xu, Z.H. Bioassay-guided isolation of DPP-4 inhibitory fractions from extracts of submerged cultured of Inonotus obliquus. Molecules 2013, 18, 1150–1161. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.K.; Lin, S.R.; Chang, C.H.; Tsai, M.J.; Lee, D.N.; Weng, C.F. Natural phenolic compounds potentiate hypoglycemia via inhibition of Dipeptidyl peptidase IV. Sci. Rep. 2019, 9, 15585. [Google Scholar] [CrossRef] [PubMed]
- Kalhotra, P.; Chittepu, V.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Structure(-)Activity Relationship and Molecular Docking of Natural Product Library Reveal Chrysin as a Novel Dipeptidyl Peptidase-4 (DPP-4) Inhibitor: An Integrated In Silico and In Vitro Study. Molecules 2018, 23, 1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.R.; Kim, H.Y.; Choi, I.; Kim, J.B.; Jin, C.H.; Han, A.R. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses. Molecules 2018, 23, 1998. [Google Scholar] [CrossRef] [Green Version]
- Abdel Motaal, A.; Salem, H.H.; Almaghaslah, D.; Alsayari, A.; Bin Muhsinah, A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Shati, A.A.; El-Askary, H. Flavonol Glycosides: In Vitro Inhibition of DPPIV, Aldose Reductase and Combating Oxidative Stress are Potential Mechanisms for Mediating the Antidiabetic Activity of Cleome droserifolia. Molecules 2020, 25, 5864. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejia, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef]
- Mojica, L.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes, hypertension and oxidative stress. J. Sci. Food Agric. 2017, 97, 2401–2410. [Google Scholar] [CrossRef]
- Hatanaka, T.; Uraji, M.; Fujita, A.; Kawakami, K. Anti-oxidation activities of rice-derived peptides and their inhibitory effects on dipeptidylpeptidase-IV. Int. J. Pept. Res. Ther. 2015, 21, 479–485. [Google Scholar] [CrossRef]
- Hatanaka, T.; Inoue, Y.; Arima, J.; Kumagai, Y.; Usuki, H.; Kawakami, K.; Kimura, M.; Mukaihara, T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012, 134, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Velarde-Salcedo, A.J.; Barrera-Pacheco, A.; Lara-Gonzalez, S.; Montero-Moran, G.M.; Diaz-Gois, A.; de Mejia, E.G.; de la Rosa, A.P.B. In vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem. 2013, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Peptides Derived from Soy and Lupin Protein as Dipeptidyl-Peptidase IV Inhibitors: In Vitro Biochemical Screening and in Silico Molecular Modeling Study. J. Agric. Food Chem. 2016, 64, 9601–9606. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Salas, P.A.; Montero-Moran, G.M.; Martinez-Cuevas, P.P.; Gonzalez, C.; de la Rosa, A.P.B. Characterization of antidiabetic and antihypertensive properties of canary seed (Phalaris canariensis L.) peptides. J. Agric. Food Chem. 2014, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides 2012, 35, 114–121. [Google Scholar] [CrossRef]
- Li-Chan, E.C.; Hunag, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Peptides derived from atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Purification and identification of dipeptidyl peptidase (DPP) IV inhibitory peptides from the macroalga Palmaria palmata. Food Chem. 2015, 172, 400–406. [Google Scholar] [CrossRef] [PubMed]

| S.No. | DDP-4 Inhibitor | Brand Name | Year of Approval |
|---|---|---|---|
| 1. | Sitagliptin | Januvia | 2006 |
| 2. | Vildagliptin | Galvus | 2007 |
| 3. | Saxagliptin | Onglyza | 2009 |
| 4. | Alogliptin | Nesina and Vipidia | 2010 |
| 5. | Linagliptin | Tradjenta, Trajenta | 2011 |
| 6. | Anagliptin | Suiny | 2012 |
| 7. | Gemigliptin | Zemiglo | 2012 |
| 8. | Teneligliptin | Tenelia | 2012 |
| 9. | Evogliptin | Suganon | 2015 |
| 10. | Omarigliptin | Marizev | 2015 |
| 11. | Trelagliptin | Zafatek | 2015 |
| 12. | Gosogliptin | Satyor | 2016 |
| S.No. | Plant | Peptide Sequence | IC50 Value | References |
|---|---|---|---|---|
| 1. | Phaseolus vulgaris | KTYGL | 0.03 mg DW/mL | [124] |
| KKSSG | 0.64 mg DW/mL | [124] | ||
| GGGLHK | 0.61 mg DW/mL | [124] | ||
| CPGNK | 0.87 mg DW/mL | [124] | ||
| 2. | Oryza sativa | IP | 2.3 ± 0.1 mg/ml | [126] |
| 3. | Glycine max | IAVPTGVA | 106 µM | [128] |
| 4. | Lupinus albus | LTFPGSAED | 228 µM | [128] |
| 5. | Palmaria palmata | ILAP | 43.40 µM | [133] |
| LLAP | 53.67 µM | [133] | ||
| MAGVDHI | 159.37 µM | [133] | ||
| 6. | Avena sativa | LQAFEPLR | 103.5 µM | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, S.; Lee, E.-J.; Ahmad, K.; Ahmad, S.-S.; Lim, J.-H.; Choi, I. A Comprehensive Review and Perspective on Natural Sources as Dipeptidyl Peptidase-4 Inhibitors for Management of Diabetes. Pharmaceuticals 2021, 14, 591. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060591
Shaikh S, Lee E-J, Ahmad K, Ahmad S-S, Lim J-H, Choi I. A Comprehensive Review and Perspective on Natural Sources as Dipeptidyl Peptidase-4 Inhibitors for Management of Diabetes. Pharmaceuticals. 2021; 14(6):591. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060591
Chicago/Turabian StyleShaikh, Sibhghatulla, Eun-Ju Lee, Khurshid Ahmad, Syed-Sayeed Ahmad, Jeong-Ho Lim, and Inho Choi. 2021. "A Comprehensive Review and Perspective on Natural Sources as Dipeptidyl Peptidase-4 Inhibitors for Management of Diabetes" Pharmaceuticals 14, no. 6: 591. https://0-doi-org.brum.beds.ac.uk/10.3390/ph14060591