A New Class of PSMA-617-Based Hybrid Molecules for Preoperative Imaging and Intraoperative Fluorescence Navigation of Prostate Cancer
Abstract
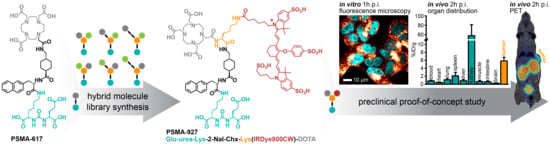
:1. Introduction
2. Results
2.1. Synthesis, Radiolabeling, and Spectral Properties
2.2. In Vitro Evaluation: PSMA-Specific Binding and Internalization Profile
2.3. In Vivo Proof-of-Concept Study: PSMA-Mediated Specific Uptake in Xenograft Tumors
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis, Radiolabeling, and Determination of Lipophilicity
4.2. Spectral Properties
4.3. Cell Binding and Internalization Properties
4.4. Confocal Microscopy
4.5. Cytotoxicity Study
4.6. Biodistribution and Preclinical Proof-of-Concept Study
4.7. Statistical Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benesova, M.; Schafer, M.; Bauder-Wust, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Hetzheim, H.; Kratochwil, C.; Benesova, M.; Eder, M.; Neels, O.C.; Eisenhut, M.; Kubler, W.; Holland-Letz, T.; Giesel, F.L.; et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J. Nucl. Med. 2015, 56, 1697–1705. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Eder, M.; Afshar-Oromieh, A.; Benesova, M.; Mier, W.; Kopka, K.; Haberkorn, U. [(1)(7)(7)Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 987–988. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; den Broeck, T.V.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Weckermann, D.; Dorn, R.; Trefz, M.; Wagner, T.; Wawroschek, F.; Harzmann, R. Sentinel lymph node dissection for prostate cancer: Experience with more than 1,000 patients. J. Urol. 2007, 177, 916–920. [Google Scholar] [CrossRef]
- Mattei, A.; Fuechsel, F.G.; Bhatta Dhar, N.; Warncke, S.H.; Thalmann, G.N.; Krause, T.; Studer, U.E. The template of the primary lymphatic landing sites of the prostate should be revisited: Results of a multimodality mapping study. Eur. Urol. 2008, 53, 118–125. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Pullambhatla, M.; Byun, Y.; Nimmagadda, S.; Foss, C.A.; Green, G.; Fox, J.J.; Lupold, S.E.; Mease, R.C.; Pomper, M.G. Sequential SPECT and optical imaging of experimental models of prostate cancer with a dual modality inhibitor of the prostate-specific membrane antigen. Angew. Chem. Int. Ed. Engl. 2011, 50, 9167–9170. [Google Scholar] [CrossRef]
- Schottelius, M.; Wurzer, A.; Wissmiller, K.; Beck, R.; Koch, M.; Gorpas, D.; Notni, J.; Buckle, T.; van Oosterom, M.N.; Steiger, K.; et al. Synthesis and Preclinical Characterization of the PSMA-Targeted Hybrid Tracer PSMA-I&F for Nuclear and Fluorescence Imaging of Prostate Cancer. J. Nucl. Med. 2019, 60, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Baranski, A.C.; Schafer, M.; Bauder-Wust, U.; Roscher, M.; Schmidt, J.; Stenau, E.; Simpfendorfer, T.; Teber, D.; Maier-Hein, L.; Hadaschik, B.; et al. PSMA-11-Derived Dual-Labeled PSMA Inhibitors for Preoperative PET Imaging and Precise Fluorescence-Guided Surgery of Prostate Cancer. J. Nucl. Med. 2018, 59, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Eder, A.C.; Schaefer, M.; Schmidt, J.; Bauder-Wuest, U.; Roscher, M.; Leotta, K.; Haberkorn, U.; Kopka, K.; Eder, M. Rational Linker Design to Accelerate Excretion and Reduce Background Uptake of Peptidomimetic PSMA-Targeting Hybrid Molecules. J. Nucl. Med. 2021, 62, 1461–1467. [Google Scholar] [CrossRef]
- Eder, A.C.; Omrane, M.A.; Stadlbauer, S.; Roscher, M.; Khoder, W.Y.; Gratzke, C.; Kopka, K.; Eder, M.; Meyer, P.T.; Jilg, C.A.; et al. The PSMA-11-derived hybrid molecule PSMA-914 specifically identifies prostate cancer by preoperative PET/CT and intraoperative fluorescence imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2057–2058. [Google Scholar] [CrossRef]
- Muckle, T.J. Plasma proteins binding of indocyanine green. Biochem. Med. 1976, 15, 17–21. [Google Scholar] [CrossRef]
- Colyer, C. Noncovalent labeling of proteins in capillary electrophoresis with laser-induced fluorescence detection. Cell Biochem. Biophys. 2000, 33, 323–337. [Google Scholar] [CrossRef]
- Ohnishi, S.; Lomnes, S.J.; Laurence, R.G.; Gogbashian, A.; Mariani, G.; Frangioni, J.V. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol. Imaging 2005, 4, 172–181. [Google Scholar] [CrossRef]
- Schafer, M.; Bauder-Wust, U.; Leotta, K.; Zoller, F.; Mier, W.; Haberkorn, U.; Eisenhut, M.; Eder, M. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. EJNMMI Res. 2012, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Benesova, M.; Bauder-Wust, U.; Schafer, M.; Klika, K.D.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Linker Modification Strategies To Control the Prostate-Specific Membrane Antigen (PSMA)-Targeting and Pharmacokinetic Properties of DOTA-Conjugated PSMA Inhibitors. J. Med. Chem. 2016, 59, 1761–1775. [Google Scholar] [CrossRef]
- Matthias, J.; Engelhardt, J.; Schafer, M.; Bauder-Wust, U.; Meyer, P.T.; Haberkorn, U.; Eder, M.; Kopka, K.; Hell, S.W.; Eder, A.C. Cytoplasmic Localization of Prostate-Specific Membrane Antigen Inhibitors May Confer Advantages for Targeted Cancer Therapies. Cancer Res. 2021, 81, 2234–2245. [Google Scholar] [CrossRef]
- Rajasekaran, S.A.; Anilkumar, G.; Oshima, E.; Bowie, J.U.; Liu, H.; Heston, W.; Bander, N.H.; Rajasekaran, A.K. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol. Biol. Cell. 2003, 14, 4835–4845. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Toriyabe, Y.; Kazak, M.; Berkman, C.E. Pseudoirreversible inhibition of prostate-specific membrane antigen by phosphoramidate peptidomimetics. Biochemistry 2008, 47, 12658–12660. [Google Scholar] [CrossRef]
- Cardinale, J.; Schafer, M.; Benesova, M.; Bauder-Wust, U.; Leotta, K.; Eder, M.; Neels, O.C.; Haberkorn, U.; Giesel, F.L.; Kopka, K. Preclinical Evaluation of (18)F-PSMA-1007, a New Prostate-Specific Membrane Antigen Ligand for Prostate Cancer Imaging. J. Nucl. Med. 2017, 58, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Eder, M.; Schafer, M.; Bauder-Wust, U.; Hull, W.E.; Wangler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef]
- Gorlitz, F.H.P.; Falk, H.J.; Kastrup, L.; Engelhardt, J.; Hell, S.W. A STED Microscope Designed for Routine Biomedical Applications. Prog. Electromagn. Res. 2014, 147, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]





| Compound | Specifically Cell Surface Bound [%AR/105 Cells] † | Specifically Internalized [%AR/105 Cells] † | ||
|---|---|---|---|---|
| PSMA-921 | 7.06 ± 3.32 | 15.82 ± 6.18 | 4.83 ± 0.93 | 4.03 ± 0.95 |
| PSMA-922 | 6.33 ± 1.53 | 13.74 ± 5.75 | 6.93 ± 1.29 | 5.78 ± 1.32 |
| PSMA-923 | 9.82 ± 0.67 | 21.55 ± 9.24 | 7.14 ± 1.95 | 5.95 ± 1.99 |
| PSMA-924 | 1.11 ± 0.81 | 1.11 ± 0.94 | 4.64 ± 1.23 | 3.87 ± 1.76 |
| PSMA-925 | 2.67 ± 0.95 | 3.03 ± 2.17 | 8.12 ± 3.59 | 6.77 ± 3.66 |
| PSMA-926 | 10.12 ± 4.85 | 11.86 ± 5.31 | 8.59 ± 2.57 | 7.16 ± 2.63 |
| PSMA-927 | 25.51 ± 9.73 | 27.64 ± 12.80 | 15.87 ± 5.46 | 13.22 ± 5.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eder, A.-C.; Matthias, J.; Schäfer, M.; Schmidt, J.; Steinacker, N.; Bauder-Wüst, U.; Domogalla, L.-C.; Roscher, M.; Haberkorn, U.; Eder, M.; et al. A New Class of PSMA-617-Based Hybrid Molecules for Preoperative Imaging and Intraoperative Fluorescence Navigation of Prostate Cancer. Pharmaceuticals 2022, 15, 267. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15030267
Eder A-C, Matthias J, Schäfer M, Schmidt J, Steinacker N, Bauder-Wüst U, Domogalla L-C, Roscher M, Haberkorn U, Eder M, et al. A New Class of PSMA-617-Based Hybrid Molecules for Preoperative Imaging and Intraoperative Fluorescence Navigation of Prostate Cancer. Pharmaceuticals. 2022; 15(3):267. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15030267
Chicago/Turabian StyleEder, Ann-Christin, Jessica Matthias, Martin Schäfer, Jana Schmidt, Nils Steinacker, Ulrike Bauder-Wüst, Lisa-Charlotte Domogalla, Mareike Roscher, Uwe Haberkorn, Matthias Eder, and et al. 2022. "A New Class of PSMA-617-Based Hybrid Molecules for Preoperative Imaging and Intraoperative Fluorescence Navigation of Prostate Cancer" Pharmaceuticals 15, no. 3: 267. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15030267