Pharmacokinetic Imaging Using 99mTc-Mebrofenin to Untangle the Pattern of Hepatocyte Transporter Disruptions Induced by Endotoxemia in Rats
Abstract
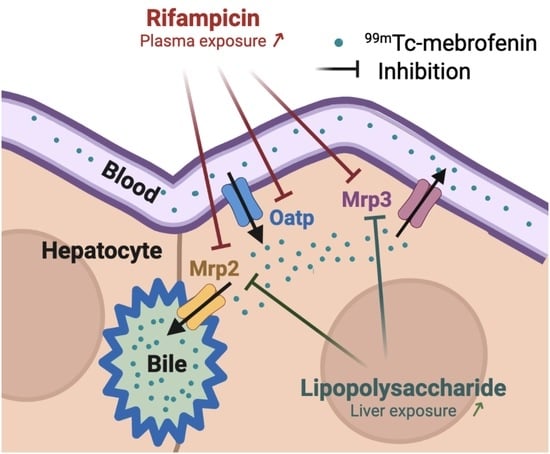
:1. Introduction
2. Results
2.1. Quantitative Transcriptomics
2.2. 99mTc-Mebrofenin Imaging
3. Discussion
4. Materials and Methods
4.1. Chemicals and Radiochemicals
4.2. Animals
4.3. Transcriptomics in LPS-Treated Rats
4.4. 99mTc-Mebrofenin Imaging
4.5. Imaging Data Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.; Fasano, A.; Miller, G.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Hermann, M. Immunological Response as a Source to Variability in Drug Metabolism and Transport. Front. Pharmacol. 2012, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanke-Labesque, F.; Gautier-Veyret, E.; Chhun, S.; Guilhaumou, R. Inflammation Is a Major Regulator of Drug Metabolizing Enzymes and Transporters: Consequences for the Personalization of Drug Treatment. Pharmacol. Ther. 2020, 215, 107627. [Google Scholar] [CrossRef] [PubMed]
- Dunvald, A.; Järvinen, E.; Mortensen, C.; Stage, T. Clinical and Molecular Perspectives on Inflammation-Mediated Regulation of Drug Metabolism and Transport. Clin. Pharmacol. Ther. 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/34605009/ (accessed on 3 March 2022). [CrossRef]
- Jetter, A.; Kullak-Ublick, G.A. Drugs and Hepatic Transporters: A Review. Pharmacol. Res. 2020, 154, 104234. [Google Scholar] [CrossRef]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs and OCTs: The Organic Anion and Cation Transporters of the SLCO and SLC22A Gene Superfamilies. Br. J. Pharmacol. 2012, 165, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.; Taskar, K.; Zamek-Gliszczynski, M. Importance of Hepatic Transporters in Clinical Disposition of Drugs and Their Metabolites. J. Clin. Pharmacol. 2016, 56, S23–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evers, R.; Piquette-Miller, M.; Polli, J.W.; Russel, F.G.M.; Sprowl, J.A.; Tohyama, K.; Ware, J.A.; de Wildt, S.N.; Xie, W.; Brouwer, K.L.R. Disease-Associated Changes in Drug Transporters May Impact the Pharmacokinetics and/or Toxicity of Drugs: A White Paper from the International Transporter Consortium. Clin. Pharmacol. Ther. 2018, 104, 900–915. [Google Scholar] [CrossRef]
- Diao, L.; Li, N.; Brayman, T.; Hotz, K.; Lai, Y. Regulation of MRP2/ABCC2 and BSEP/ABCB11 Expression in Sandwich Cultured Human and Rat Hepatocytes Exposed to Inflammatory Cytokines TNF-α, IL-6, and IL-1β. J. Biol. Chem. 2010, 285, 31185–31192. [Google Scholar] [CrossRef] [Green Version]
- Cherrington, N.; Slitt, A.; Li, N.; Klaassen, C. Lipopolysaccharide-Mediated Regulation of Hepatic Transporter MRNA Levels in Rats. Drug Metab. Dispos. 2004, 32, 734–741. [Google Scholar] [CrossRef] [Green Version]
- Kubitz, R.; Wettstein, M.; Warskulat, U.; Häussinger, D. Regulation of the Multidrug Resistance Protein 2 in the Rat Liver by Lipopolysaccharide and Dexamethasone. Gastroenterology 1999, 116, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Donner, M.G.; Warskulat, U.; Saha, N.; Häussinger, D. Enhanced Expression of Basolateral Multidrug Resistance Protein Isoforms Mrp3 and Mrp5 in Rat Liver by LPS. Biol. Chem. 2004, 385, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Saib, S.; Delavenne, X. Inflammation Induces Changes in the Functional Expression of P-Gp, BCRP, and MRP2: An Overview of Different Models and Consequences for Drug Disposition. Pharmaceutics 2021, 13, 1544. [Google Scholar] [CrossRef] [PubMed]
- Corsini, A.; Bortolini, M. Drug-Induced Liver Injury: The Role of Drug Metabolism and Transport. J. Clin. Pharmacol. 2013, 53, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, K.M.; Huang, S.-M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane Transporters in Drug Development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [PubMed]
- Karlgren, M.; Vildhede, A.; Norinder, U.; Wisniewski, J.; Kimoto, E.; Lai, Y.; Haglund, U.; Artursson, P. Classification of Inhibitors of Hepatic Organic Anion Transporting Polypeptides (OATPs): Influence of Protein Expression on Drug–Drug Interactions. J. Med. Chem. 2012, 55, 4740–4763. [Google Scholar] [CrossRef]
- Matsson, P.; Pedersen, J.; Norinder, U.; Bergström, C.; Artursson, P. Identification of Novel Specific and General Inhibitors of the Three Major Human ATP-Binding Cassette Transporters P-Gp, BCRP and MRP2 Among Registered Drugs. Pharm. Res. 2009, 26, 1816–1831. [Google Scholar] [CrossRef]
- Lengyel, G.; Veres, Z.; Tugyi, R.; Vereczkey, L.; Molnár, T.; Glavinas, H.; Krajcsi, P.; Jemnitz, K. Modulation of Sinusoidal and Canalicular Elimination of Bilirubin-Glucuronides by Rifampicin and Other Cholestatic Drugs in a Sandwich Culture of Rat Hepatocytes. Hepatol. Res. 2008, 38, 300–309. [Google Scholar] [CrossRef]
- Ishida, K.; Ullah, M.; Tóth, B.; Juhasz, V.; Unadkat, J. Transport Kinetics, Selective Inhibition, and Successful Prediction of In Vivo Inhibition of Rat Hepatic Organic Anion Transporting Polypeptides. Drug Metab. Dispos. 2018, 46, 1251–1258. [Google Scholar] [CrossRef]
- Takashima, T.; Kitamura, S.; Wada, Y.; Tanaka, M.; Shigihara, Y.; Ishii, H.; Ijuin, R.; Shiomi, S.; Nakae, T.; Watanabe, Y.; et al. PET Imaging–Based Evaluation of Hepatobiliary Transport in Humans with (15R)-11C-TIC-Me. J. Nucl. Med. 2012, 53, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Ghibellini, G.; Leslie, E.; Brouwer, K. Methods to Evaluate Biliary Excretion of Drugs in Humans: An Updated Review. Mol. Pharm. 2006, 3, 198–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, A.; Moorthy, B.; Ghose, R. Drug Disposition in Pathophysiological Conditions. Curr. Drug Metab. 2012, 13, 1327–1344. [Google Scholar] [PubMed] [Green Version]
- Marie, S.; Hernandez-Lozano, I.; Langer, O.; Tournier, N. Repurposing 99mTc-Mebrofenin as a Probe for Molecular Imaging of Hepatocyte Transporters. J. Nucl. Med. 2021, 62, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Neyt, S.; Huisman, M.; Vanhove, C.; Man, H.; Vliegen, M.; Moerman, L.; Dumolyn, C.; Mannens, G.; Vos, F. In Vivo Visualization and Quantification of (Disturbed) Oatp-Mediated Hepatic Uptake and Mrp2-Mediated Biliary Excretion of 99mTc-Mebrofenin in Mice. J. Nucl. Med. 2013, 54, 624–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargava, K.; Joseph, B.; Ananthanarayanan, M.; Balasubramaniyan, N.; Tronco, G.; Palestro, C.; Gupta, S. Adenosine Triphosphate–Binding Cassette Subfamily C Member 2 Is the Major Transporter of the Hepatobiliary Imaging Agent 99mTc-Mebrofenin. J. Nucl. Med. 2009, 50, 1140–1146. [Google Scholar] [CrossRef] [Green Version]
- Marie, S.; Hernández-Lozano, I.; Breuil, L.; Truillet, C.; Hu, S.; Sparreboom, A.; Tournier, N.; Langer, O. Imaging-Based Characterization of a Slco2b1(-/-) Mouse Model Using [11C]Erlotinib and [99mTc]Mebrofenin as Probe Substrates. Pharmaceutics 2021, 13, 918. [Google Scholar] [CrossRef]
- Pfeifer, N.; Goss, S.; Swift, B.; Ghibellini, G.; Ivanovic, M.; Heizer, W.; Gangarosa, L.; Brouwer, K. Effect of Ritonavir on 99mTechnetium–Mebrofenin Disposition in Humans: A Semi-PBPK Modeling and In Vitro Approach to Predict Transporter-Mediated DDIs. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e20. [Google Scholar] [CrossRef]
- Ali, I.; Slizgi, J.; Kaullen, J.; Ivanovic, M.; Niemi, M.; Stewart, P.; Barritt, A.; Brouwer, K. Transporter-Mediated Alterations in Patients with NASH Increase Systemic and Hepatic Exposure to an OATP and MRP2 Substrate. Clin. Pharmacol. Ther. 2017, 104, 749–756. [Google Scholar] [CrossRef]
- Marie, S.; Hernández-Lozano, I.; Breuil, L.; Saba, W.; Novell, A.; Gennisson, J.; Langer, O.; Truillet, C.; Tournier, N. Validation of Pharmacological Protocols for Targeted Inhibition of Canalicular MRP2 Activity in Hepatocytes Using [99mTc]Mebrofenin Imaging in Rats. Pharmaceutics 2020, 12, 486. [Google Scholar] [CrossRef]
- Elferink, M.; Olinga, P.; Draaisma, A.; Merema, M.; Faber, K.; Slooff, M.; Meijer, D.; Groothuis, G. LPS-Induced Downregulation of MRP2 and BSEP in Human Liver Is Due to a Posttranscriptional Process. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 287, G1008–G1016. [Google Scholar] [CrossRef]
- Tournier, N.; Stieger, B.; Langer, O. Imaging Techniques to Study Drug Transporter Function In Vivo. Pharmacol. Ther. 2018, 189, 104–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, C.; Huang, L. Xenobiotic Receptors in Mediating the Effect of Sepsis on Drug Metabolism. Acta Pharm. Sin. B 2020, 10, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, V.; Aithal, G. Hepatotoxicity Related to Anti-Tuberculosis Drugs: Mechanisms and Management. J. Clin. Exp. Hepatol. 2013, 3, 37–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segovia-Zafra, A.; Di Zeo-Sánchez, D.; López-Gómez, C.; Pérez-Valdés, Z.; García-Fuentes, E.; Andrade, R.; Lucena, M.; Villanueva-Paz, M. Preclinical Models of Idiosyncratic Drug-Induced Liver Injury (IDILI): Moving towards Prediction. Acta Pharm. Sin. B 2021, 11, 3685–3726. [Google Scholar] [CrossRef] [PubMed]
- Roughneen, P.T.; Kumar, S.C.; Pellis, N.R.; Rowlands, B.J. Endotoxemia and Cholestasis. Surg. Gynecol. Obstet. 1988, 167, 205–210. [Google Scholar]
- Chand, N.; Sanyal, A. Sepsis-Induced Cholestasis. Hepatology 2007, 45, 230–241. [Google Scholar] [CrossRef]
- Schmith, V.; Foss, J. Effects of Inflammation on Pharmacokinetics/Pharmacodynamics: Increasing Recognition of Its Contribution to Variability in Response. Clin. Pharmacol. Ther. 2008, 83, 809–811. [Google Scholar] [CrossRef]
- Le Vee, M.; Jouan, E.; Noel, G.; Stieger, B.; Fardel, O. Polarized Location of SLC and ABC Drug Transporters in Monolayer-Cultured Human Hepatocytes. Toxicol. In Vitro 2015, 29, 938–946. [Google Scholar] [CrossRef] [Green Version]
- Hernández Lozano, I.; Karch, R.; Bauer, M.; Blaickner, M.; Matsuda, A.; Wulkersdorfer, B.; Hacker, M.; Zeitlinger, M.; Langer, O. Towards Improved Pharmacokinetic Models for the Analysis of Transporter-Mediated Hepatic Disposition of Drug Molecules with Positron Emission Tomography. AAPS J. 2019, 21, 61. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marie, S.; Hernández-Lozano, I.; Le Vée, M.; Breuil, L.; Saba, W.; Goislard, M.; Goutal, S.; Truillet, C.; Langer, O.; Fardel, O.; et al. Pharmacokinetic Imaging Using 99mTc-Mebrofenin to Untangle the Pattern of Hepatocyte Transporter Disruptions Induced by Endotoxemia in Rats. Pharmaceuticals 2022, 15, 392. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15040392
Marie S, Hernández-Lozano I, Le Vée M, Breuil L, Saba W, Goislard M, Goutal S, Truillet C, Langer O, Fardel O, et al. Pharmacokinetic Imaging Using 99mTc-Mebrofenin to Untangle the Pattern of Hepatocyte Transporter Disruptions Induced by Endotoxemia in Rats. Pharmaceuticals. 2022; 15(4):392. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15040392
Chicago/Turabian StyleMarie, Solène, Irene Hernández-Lozano, Marc Le Vée, Louise Breuil, Wadad Saba, Maud Goislard, Sébastien Goutal, Charles Truillet, Oliver Langer, Olivier Fardel, and et al. 2022. "Pharmacokinetic Imaging Using 99mTc-Mebrofenin to Untangle the Pattern of Hepatocyte Transporter Disruptions Induced by Endotoxemia in Rats" Pharmaceuticals 15, no. 4: 392. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15040392