Anti-Diabetic Effects of Allium hookeri Extracts Prepared by Different Methods in Type 2 C57BL/J-db/db Mice
Abstract
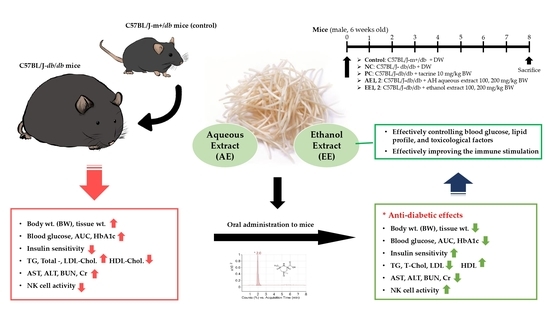
:1. Introduction
2. Results
2.1. Concentration of Cycloalliin
2.2. Effects of A. hookeri Extracts on Body and Organ/Tissue Weights
2.3. Effects of A. hookeri Extracts on Fasting Blood Glucose
2.4. Effects of A. hookeri Extracts on Oral Glucose Tolerance and AUC
2.5. Effects of A. hookeri Extracts on Glycated Hemoglobin (HbA1c), Insulin, Leptin, and Adiponectin Levels
2.6. Effects of A. hookeri Extracts on Serum ALT, AST, BUN, and Cr Levels
2.7. Effects of A. hookeri Extracts on Serum Lipid Profile
2.8. Effects of A. hookeri Extracts on Histopathological Properties of Liver, Pancreas, and Kidney
2.9. Effects of A. hookeri Extracts on the NK Activity in the Blood of Diabetic Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of Extract Samples
4.2. Measuring Cycloalliin Concentration
4.3. Animals and Diets
4.4. Measuring Blood Glucose and Conducting Oral Glucose Tolerance Test (OGTT)
4.5. Blood Biochemical Analysis
4.6. Histopathological and Immunohistochemical Investigations
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roglic, G. WHO Global report on diabetes: A summary. Int. J. Noncommun. Dis. 2016, 1, 3. [Google Scholar] [CrossRef]
- Cantley, J.; Ashcroft, F.M. Q&A: Insulin secretion and type 2 diabetes: Why do β-cells fail? BMC Biology 2015, 13, 33. [Google Scholar]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its compolications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomous, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Young, J.L.; Wang, K.; Qian, Y.; Cai, L. Diabetic-induced alterations in hepatic glucose and lipid metabolism: The role of type 1 and type 2 diabetes mellitus (review). Mol. Med. Rep. 2020, 22, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Wondmkun, Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Chen, S.C.C.; Tsai, S.P.; Jhao, J.Y.; Jiang, W.K.; Tsao, C.K.; Chang, L.Y. Liver fat, hepatic enzymes, alkaline phosphatase and the risk of incident type 2 diabetes: A prospective study of 132,377 adults. Sci. Rep. 2017, 7, 4649. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Veeraraghavan, V.P.; Kim, S.; Wang, M.H. Chemical composition, antioxidant, and anti-diabetic activities of ethyl acetate fraction of Stachys riederi var. japonica (Miq.) in streptozotocin-induced type 2 diabetic mice. Food Chem. Toxicol. 2021, 155, 112374. [Google Scholar] [CrossRef]
- Shah, N.A.; Khan, M.R. Antidiabetic effect of Sida cordata in alloxan induced diabetic rats. BioMed Res. Int. 2014, 2014, 671294. [Google Scholar] [CrossRef] [Green Version]
- Giribabu, N.; Eswar Kumar, K.; Swapna Rekha, S.; Muniandy, S.; Salleh, N. Vitis vinifera (Muscat Variety) seed ethanolic extract preserves activity levels of enzymes and histology of the liver in adult male rats with diabetes. Evid.-Based Complement. Altern. Med. 2015, 2015, 542026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, U.; Khan, M.R.; Sajid, M. Antioxidant, anti-inflammatory and hypoglycemic effects of Fagonia olivieri DC on STZ-nicotinamide induced diabetic rats-in vivo and in vitro study. J. Ethnopharmacol. 2019, 242, 112038. [Google Scholar] [CrossRef] [PubMed]
- Chutani, A.; Pande, S. Correlation of serum creatinine and urea with glycemic index and duration of diabetes in Type 1 and Type 2 diabetes mellitus: A comparative study. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 914–919. [Google Scholar] [CrossRef]
- Frydrych, L.M.; Bian, G.; O’Lone, D.E.; Ward, P.A.; Delano, M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 2018, 104, 525–534. [Google Scholar] [CrossRef]
- Tiwari, S.; Pratyush, D.D.; Gahlot, A.; Singh, S.K. Sepsis in diabetes: A bad duo. Diabetes Metab. Syndr. 2011, 5, 222–227. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef]
- Hundal, R.S.; Inzucchi, S.E. Metformin: New understanding, new use. Drugs 2003, 63, 1879–1894. [Google Scholar] [CrossRef]
- Kim, N.S.; Choi, B.K.; Lee, S.H.; Jang, H.H.; Kim, J.B.; Kim, H.R.; Choe, J.S.; Cho, Y.S.; Kim, Y.S.; Yang, J.H.; et al. Effects of Allium Hookeri Extracts on Glucose Metabolism in Type II Diabetic Mice. Korean J. Pharm. 2016, 47, 158–164. [Google Scholar]
- Park, S.H.; Bae, U.J.; Choi, E.K.; Jung, S.J.; Lee, S.H.; Yang, J.H.; Chae, S.W. A randomized, double-blind, placebo-controlled crossover clinical trial to evaluate the anti-diabetic effects of Allium hookeri extract in the subjects with prediabetes. BMC Complementary Med. Ther. 2020, 20, 211. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, M.J.; Jang, J.Y.; Lee, S.H. Allium hookeri root extract inhibits adipogenesis by promoting lipolysis in high fat diet-induced obese mice. Nutrients 2019, 11, 2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, U.J.; Choi, H.; Yoon, W.C.; Kim, Y.S.; Song, B.N.; Lee, M.Y.; Park, B.R.; Lee, S.H.; Choi, J.H.; Park, S.Y. Anti-diabetic and Lipid Profile Effect of Astragalus membranaceus (Fisch.) Bunge Fermented by Aspergillus awamori in db/db Mice. Korean J. Med. Crop. Sci 2021, 29, 263–272. [Google Scholar] [CrossRef]
- Eslami, M.; Bahar, A.; Hemati, M.; Rasouli Nejad, Z.; Mehranfar, F.; Karami, S.; Yousefi, B. Dietary pattern, colonic microbiota and immunometabolism interaction: New frontiers for diabetes mellitus and related disorders. Diabet. Med. 2021, 38, e14415. [Google Scholar] [CrossRef] [PubMed]
- Shabab, S.; Gholamnezhad, Z.; Mahmoudabady, M. Protective effects of medicinal plant against diabetes induced cardiac disorder: A review. J. Ethnopharmacol. 2021, 265, 113328. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxidative Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patle, D.; Vyas, M.; Khatik, G.L. A review on natural products and herbs used in the management of diabetes. Curr. Diabetes Rev. 2021, 17, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R. Bioactive Compounds of Allium Species. In Bioactive Compounds in Underutilized Vegetables and Legumes; Reference Series in Phytochemistry; Murthy, H.N., Paek, K.Y., Eds.; Springer: Cham, Swizerland, 2020; pp. 1–20. [Google Scholar]
- Lawson, L.D.; Hunsaker, S.M. Allicin bioavailability and bioequivalence from garlic supplements and garlic foods. Nutrients 2018, 10, 812. [Google Scholar] [CrossRef] [Green Version]
- Kovarovic, J.; Bystricka, J.; Vollmannova, A.; Toth, T.; Brindza, J. Biologically valuable substances in garlic (Allium sativum L.)—A review. J. Cent. Eur. Agric. 2019, 20, 292–304. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Suh, H.J.; Han, S.H.; Hong, J.; Choi, H.S. Optimization of extraction of cycloalliin from garlic (Allium sativum L.) by using principal components analysis. Prev. Nutr. Food Sci. 2016, 21, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.W. Effect of Allium hookeri root on plasma blood glucose and fat profile levels in streptozotocin-induced diabetic rats. J. East. Asian Soc. Diet. Life 2016, 26, 481–490. [Google Scholar] [CrossRef]
- Lee, J. The hypolipidemic effect of Allium hookeri in rats fed with a high fat diet. Korean J. Community Living Sci. 2016, 27, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; No, K.; Lee, J. Anti-obesity effect of Allium hookeri leaf extract in high-fat diet-fed mice. J. Med. Food 2018, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Heo, J.S.; Choi, J.W.; Kim, G.D.; Sohn, K.H. Allium hookeri extract improves type 2 diabetes mellitus in C57BL/KSJ Db/db obese mouse via regulation of hepatic lipogenesis and glucose metabolism. J. Life Sci. 2015, 25, 1081–1090. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Chin, K.Y.; Soelaiman, I.N. Leptin, adiponectin and insulin as regulators for energy metabolism in a rat model of metabolic syndrome. Sains Malays. 2019, 48, 2701–2707. [Google Scholar] [CrossRef]
- Mansour, S.A.; Mossa, A.T. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic. Biochem. Phys. 2010, 96, 14–23. [Google Scholar] [CrossRef]
- Bamanikar, S.A.; Bamanikar, A.A.; Arora, A. Study of serum urea and creatinine in diabetic and non-diabetic patients in a tertiary teaching hospital. J. Med. Res. 2016, 2, 12–15. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, K.; Lee, S.B.; Kang, S.; Park, J.S.; Ahn, C.W.; Nam, J.S. Relationship between natural killer cell activity and glucose control in patients with type 2 diabetes and prediabetes. J. Diabetes Investig. 2019, 10, 1223–1228. [Google Scholar] [CrossRef] [Green Version]









| Control | NC | PC | AE1 | AE2 | EE1 | EE2 | |
|---|---|---|---|---|---|---|---|
| Initial body weight (g) | 21.87 ± 0.82 b | 32.53 ± 5.29 a | 33.63 ± 4.16 a | 33.42 ± 3.96 a | 33.93 ± 4.26 a | 31.73 ± 3.96 a | 33.65 ± 4.25 a |
| Final body weight (g) | 27.65 ± 0.31 b | 33.55 ± 0.82 a | 32.62 ± 3.63 a | 32.82 ± 3.82 a | 29.35 ± 3.41 a | 32.84 ± 3.78 a | 29.86 ± 3.21 a |
| Tissue weight (% of BW) | |||||||
| Liver | 3.78 ± 0.08 c | 6.28 ± 0.21 a | 5.40 ± 0.16 b | 5.25 ± 0.06 b | 5.38 ± 0.21 b | 5.42 ± 0.11 b | 5.59 ± 0.14 b |
| Kidneys | 0.98 ± 0.08 c | 1.44 ± 0.09 a | 1.01 ± 0.04 c | 1.11 ± 0.03 bc | 1.10 ± 0.07 c | 1.24 ± 0.05 ab | 1.27 ± 0.03 ab |
| Epididymal fat | 2.22 ± 0.10 d | 5.39 ± 0.15 a | 5.09 ± 0.16 ab | 5.10 ± 0.10 ab | 4.79 ± 0.17 bc | 4.91 ± 0.14 ab | 4.43 ± 0.22 c |
| Spleen | 0.27 ± 0.02 a | 0.10 ± 0.01 c | 0.13 ± 0.01 bc | 0.12 ± 0.01 bc | 0.16 ± 0.02 b | 0.15 ± 0.03 b | 0.17 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Kim, S.-H.; Lee, E.-B.; Kim, J.-S.; Jung, J.-E.; Jeong, U.-Y.; Kim, J.-H.; Jang, H.-H.; Park, S.-Y.; Kim, G.-C.; et al. Anti-Diabetic Effects of Allium hookeri Extracts Prepared by Different Methods in Type 2 C57BL/J-db/db Mice. Pharmaceuticals 2022, 15, 486. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15040486
Choi J-H, Kim S-H, Lee E-B, Kim J-S, Jung J-E, Jeong U-Y, Kim J-H, Jang H-H, Park S-Y, Kim G-C, et al. Anti-Diabetic Effects of Allium hookeri Extracts Prepared by Different Methods in Type 2 C57BL/J-db/db Mice. Pharmaceuticals. 2022; 15(4):486. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15040486
Chicago/Turabian StyleChoi, Ji-Hye, Si-Hyun Kim, Eun-Byeol Lee, Ji-Su Kim, Ji-Eeun Jung, Un-Yul Jeong, Ju-Hui Kim, Hwan-Hee Jang, Shin-Young Park, Gi-Chang Kim, and et al. 2022. "Anti-Diabetic Effects of Allium hookeri Extracts Prepared by Different Methods in Type 2 C57BL/J-db/db Mice" Pharmaceuticals 15, no. 4: 486. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15040486