Microencapsulating Alginate-Based Polymers for Probiotics Delivery Systems and Their Application
Abstract
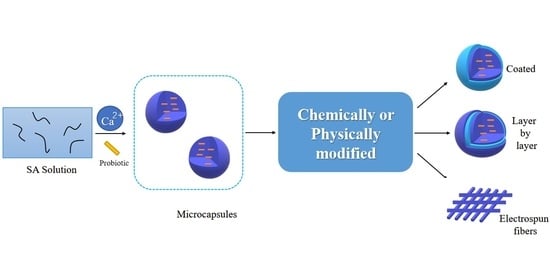
:1. Introduction
2. Alginate-Based Probiotics Delivery
2.1. Natural Alginate
2.2. Chemically Modified Alginate
2.2.1. Chitosan
2.2.2. Protein
2.2.3. Wild Sage Seed Mucilage (WSSM)
2.3. Physically Modified Alginate
2.3.1. Starch
2.3.2. Poly (Vinyl Alcohol)
2.3.3. Polystyrene
2.3.4. Carboxymethylpachymaran
3. Applications, Prospects, and Challenges
3.1. Applications of Microencapsulated Probiotics
3.2. Prospects and Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Who, F. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria—Joint FAO/WHO Expert Consultation. 2001. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 28 April 2022).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Wang, Z.; Bai, L.; Deng, J.; Zhou, Q. Biomaterial-based encapsulated probiotics for biomedical applications: Current status and future perspectives. Mater. Des. 2021, 210, 110018. [Google Scholar] [CrossRef]
- Aggarwal, N.; Breedon, A.M.E.; Davis, C.M.; Hwang, I.Y.; Chang, M.W. Engineering probiotics for therapeutic applications: Recent examples and translational outlook. Curr. Opin. Biotechnol. 2020, 65, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, A.; Behdani, R.; Kouhestani, S. Probiotics: A Comprehensive Review of Their Classification, Mode of Action and Role in Human Nutrition. Probiotics Prebiotics Hum. Nutr. Health 2016, 10, 63646. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Microencapsulating polymers for probiotics delivery systems: Preparation, characterization, and applications. Food Hydrocoll. 2021, 120, 106882. [Google Scholar] [CrossRef]
- Saxelin, M.; Tynkkynen, S.; Mattila-Sandholm, T.; de Vos, W.M. Probiotic and other functional microbes: From markets to mechanisms. Curr. Opin. Biotechnol. 2005, 16, 204–211. [Google Scholar] [CrossRef]
- Bajaj, B.K.; Claes, I.J.; Lebeer, S. Functional mechanisms of probiotics. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Harper, A.; Naghibi, M.M.; Garcha, D. The Role of Bacteria, Probiotics and Diet in Irritable Bowel Syndrome. Foods 2018, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Mego, M.; Holec, V.; Drgona, L.; Hainova, K.; Ciernikova, S.; Zajac, V. Probiotic bacteria in cancer patients undergoing chemotherapy and radiation therapy. Complement. Ther. Med. 2013, 21, 712–723. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Sidira, M.; Charalampopoulos, D.; Ypsilantis, P.; Galanis, A.; Simopoulos, C.; Kourkoutas, Y. Effect of Cell Immobilization on Properties of Presumptive Probiotics. In Proceedings of the 6th Central European Congress on Food (CEFood), Novi Sad, Serbia, 23–26 May 2012; pp. 257–268. [Google Scholar]
- Chen, Z.Y.; Guo, L.L.; Zhang, Y.Q.; Walzem, R.L.; Pendergast, J.S.; Printz, R.L.; Morris, L.C.; Matafonova, E.; Stien, X.; Kang, L.; et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J. Clin. Investig. 2014, 124, 3391–3406. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obs. Gynecol. 2015, 212, 496.e1–496.e11. [Google Scholar]
- Serban, D.E. Gastrointestinal cancers: Influence of gut microbiota, probiotics and prebiotics. Cancer Lett. 2014, 345, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Monachese, M.; Cunningham-Rundles, S.; Diaz, M.A.; Guerrant, R.; Hummelen, R.; Kemperman, R.; Kerac, M.; Kort, R.; Merenstein, D.J.; Panigrahi, P.; et al. Probiotics and prebiotics to combat enteric infections and HIV in the developing world: A consensus report. Gut Microbes 2011, 2, 198–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claes, I.J.J.; Lebeer, S.; Shen, C.; Verhoeven, T.L.A.; Dilissen, E.; De Hertogh, G.; Bullens, D.M.A.; Ceuppens, J.L.; Van Assche, G.; Vermeire, S.; et al. Impact of lipoteichoic acid modification on the performance of the probiotic Lactobacillus rhamnosus GG in experimental colitis. Clin. Exp. Immunol. 2010, 162, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Muhammad, N.; Jhun, B.H.; Yoo, J.-W. Probiotic delivery systems: A brief overview. J. Pharm. Investig. 2016, 46, 377–386. [Google Scholar] [CrossRef]
- Jiang, Z.W.; Li, M.T.; McClements, D.J.; Liu, X.B.; Liu, F.G. Recent advances in the design and fabrication of probiotic delivery systems to target intestinal inflammation. Food Hydrocoll. 2022, 125, 107438. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A.; Simal-Gandara, J. Technological strategies ensuring the safe arrival of beneficial microorganisms to the gut: From food processing and storage to their passage through the gastrointestinal tract. Food Res. Int. 2020, 129, 108852. [Google Scholar] [CrossRef]
- Feng, K.; Huang, R.-M.; Wu, R.-Q.; Wei, Y.-S.; Zong, M.-H.; Linhardt, R.J.; Wu, H. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 2020, 310, 125977. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Assembly of Protein–Polysaccharide Complexes for Delivery of Bioactive Ingredients: A Perspective Paper. J. Agric. Food Chem. 2019, 67, 1344–1352. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Xue, C.; Wei, Z. Latest developments in food-grade delivery systems for probiotics: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Microencapsulation of Saccharomyces cerevisiae into Alginate Beads: A Focus on Functional Properties of Released Cells. Foods 2020, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Hadinoto, K. Biofilm-Like Lactobacillus rhamnosus Probiotics Encapsulated in Alginate and Carrageenan Microcapsules Exhibiting Enhanced Thermotolerance and Freeze-Drying Resistance. Biomacromolecules 2013, 14, 3214–3222. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zheng, W.; Sun, Z.; Zhang, D.; Sui, K.; Shen, P.; Li, P.; Zhou, Q. Marine polysaccharide-based composite hydrogels containing fucoidan: Preparation, physicochemical characterization, and biocompatible evaluation. Int. J. Biol. Macromol. 2021, 183, 1978–1986. [Google Scholar] [CrossRef]
- He, Y.; Zhao, W.W.; Dong, Z.X.; Ji, Y.J.; Li, M.; Hao, Y.P.; Zhang, D.M.; Yuan, C.Q.; Deng, J.; Zhao, P.; et al. A biodegradable antibacterial alginate/carboxymethyl chitosan/Kangfuxin sponges for promoting blood coagulation and full-thickness wound healing. Int. J. Biol. Macromol. 2021, 167, 182–192. [Google Scholar] [CrossRef]
- Hao, Y.P.; Zhao, W.W.; Zhang, L.Y.; Zeng, X.; Sun, Z.Y.; Zhang, D.M.; Shen, P.L.; Li, Z.X.; Han, Y.T.; Li, P.F.; et al. Bio-multifunctional alginate/chitosan/fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- De Vos, P.; Lazarjani, H.A.; Poncelet, D.; Faas, M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv. Drug Deliv. Rev. 2014, 67–68, 15–34. [Google Scholar] [CrossRef]
- Schmidt, J.J.; Rowley, J.; Kong, H.J. Hydrogels used for cell-based drug delivery. J. Biomed. Mater. Res. Part A 2008, 87, 1113–1122. [Google Scholar] [CrossRef]
- Chandramouli, V.; Kailasapathy, K.; Peiris, P.; Jones, M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J. Microbiol. Methods 2004, 56, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2015, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Q.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y.P. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Braccini, I.; Grasso, R.P.; Pérez, S. Conformational and configurational features of acidic polysaccharides and their interactions with calcium ions: A molecular modeling investigation. Carbohydr. Res. 1999, 317, 119–130. [Google Scholar] [CrossRef]
- Simpson, N.E.; Stabler, C.L.; Simpson, C.P.; Sambanis, A.; Constantinidis, I. The role of the CaCl2–guluronic acid interaction on alginate encapsulated βTC3 cells. Biomaterials 2004, 25, 2603–2610. [Google Scholar] [CrossRef]
- Li, Y.; Feng, C.; Li, J.; Mu, Y.Z.; Liu, Y.; Kong, M.; Cheng, X.J.; Chen, X.G. Construction of multilayer alginate hydrogel beads for oral delivery of probiotics cells. Int. J. Biol. Macromol. 2017, 105, 924–930. [Google Scholar] [CrossRef]
- Qi, W.T.; Liang, X.X.; Yun, T.T.; Guo, W.Q. Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. J. Food Sci. Technol. 2019, 56, 1398–1404. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, K.F.D.; de Oliveira, K.A.R.; de Souza, E.L. Application of Potentially Probiotic Fruit-Derived Lactic Acid Bacteria Loaded into Sodium Alginate Coatings to Control Anthracnose Development in Guava and Mango During Storage. Probiotics Antimicrob Proteins 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Vega-Carranza, A.S.; Cervantes-Chávez, J.A.; Luna-Bárcenas, G.; Luna-González, A.; Diarte-Plata, G.; Nava-Mendoza, R.; Rodríguez-Morales, J.A.; Escamilla-Montes, R.; Pool, H. Alginate microcapsules as delivery and protective systems of Bacillus licheniformis in a simulated shrimp’s digestive tract. Aquaculture 2021, 540, 736675. [Google Scholar] [CrossRef]
- Iurciuc, C.E.; Peptu, C.A.; Savin, A.; Atanase, L.-I.L.-I.; Souidi, K.; Mackenzie, G.; Martin, P.; Riess, G.; Popa, M. Microencapsulation of Baker’s Yeast in Gellan Gum Beads Used in Repeated Cycles of Glucose Fermentation. Int. J. Polym. Sci. 2017, 2017, 7610420. [Google Scholar] [CrossRef] [Green Version]
- Duarte, J.C.; Rodrigues, J.A.R.; Moran, P.J.S.; Valença, G.P.; Nunhez, J.R. Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express 2013, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Gallo, M.; Bevilacqua, A.; Speranza, B.; Sinigaglia, M.; Corbo, M.R. Alginate beads and apple pieces as carriers for Saccharomyces cerevisiae var. boulardii, as representative of yeast functional starter cultures. Int. J. Food Sci. Tech. 2014, 49, 2092–2100. [Google Scholar] [CrossRef]
- Suvarna, S.; Dsouza, J.; Ragavan, M.L.; Das, N. Potential probiotic characterization and effect of encapsulation of probiotic yeast strains on survival in simulated gastrointestinal tract condition. Food Sci. Biotechnol. 2018, 27, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, Z.; Xue, C. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5345–5369. [Google Scholar] [CrossRef]
- Gobi, N.; Vaseeharan, B.; Chen, J.-C.; Rekha, R.; Vijayakumar, S.; Anjugam, M.; Iswarya, A. Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish. Immunol. 2018, 74, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.F.; Wang, Y.; Dong, H.B.; Ding, X.; Liu, Q.S.; Li, H.; Zhang, J.S.; Xiong, D.L. Changes in the Intestine Microbial, Digestive, and Immune-Related Genes of Litopenaeus vannamei in Response to Dietary Probiotic Clostridium butyricum Supplementation. Front. Microbiol. 2018, 9, 2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Cheng, Y.Z.; Chen, X.Y.; Liu, Z.P.; Long, X.H. Effects of small peptides, probiotics, prebiotics, and synbiotics on growth performance, digestive enzymes, and oxidative stress in orange-spotted grouper, Epinephelus coioides, juveniles reared in artificial seawater. Chin. J. Oceanol. Limnol. 2017, 35, 89–97. [Google Scholar] [CrossRef]
- Oberoi, K.; Tolun, A.; Altintas, Z.; Sharma, S. Effect of Alginate-Microencapsulated Hydrogels on the Survival of Lactobacillus rhamnosus under Simulated Gastrointestinal Conditions. Foods 2021, 10, 1999. [Google Scholar] [CrossRef]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef]
- Hansen, L.T.; Allan-Wojtas, P.M.; Jin, Y.-L.; Paulson, A.T. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiol. 2002, 19, 35–45. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Gandomi, H.; Misaghi, A.; Bokaei, S.; Noori, N. The effect of alginate and chitosan concentrations on some properties of chitosan-coated alginate beads and survivability of encapsulated Lactobacillus rhamnosus in simulated gastrointestinal conditions and during heat processing. J. Sci. Food Agric. 2014, 94, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Lu, Y.; Zhang, T.; Xie, J.; Han, S.; Zhang, S.; Fei, Y.; Ling, Z.; Wu, J.; Hu, Y.; et al. Improved functionality of Ligilactobacillus salivarius Li01 in alleviating colonic inflammation by layer-by-layer microencapsulation. Npj Biofilms Microbiomes 2021, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-Layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef] [Green Version]
- Krunić, T.Z.; Bulatović, M.L.J.; Obradović, N.S.; Vukašinović-Sekulić, M.S.; Rakin, M.B. Effect of immobilisation materials on viability and fermentation activity of dairy starter culture in whey-based substrate. J. Sci. Food Agric. 2016, 96, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Song, C.E.; Shim, H.H.; Kuppusamy, P.; Jeong, Y.-I.; Lee, K.D. Potential Sustainable Properties of Microencapsulated Endophytic Lactic Acid Bacteria (KCC-42) in In-Vitro Simulated Gastrointestinal Juices and Their Fermentation Quality of Radish Kimchi. BioMed Res. Int. 2018, 2018, 6015243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.; Wu, J.H.; Zhang, J.L.; Wang, T.; Zhang, X.D.; Shao, L.; Chen, D.J.; Wang, J. Extending Viability of Bifidobacterium longum in Chitosan-Coated Alginate Microcapsules Using Emulsification and Internal Gelation Encapsulation Technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; del Carmen Villarán, M.D. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Wang, M.; Zang, Y.; Hong, K.; Zhao, X.; Yu, C.; Liu, D.; An, Z.; Wang, L.; Yue, W.; Nie, G. Preparation of pH-sensitive carboxymethyl cellulose/chitosan/alginate hydrogel beads with reticulated shell structure to deliver Bacillus subtilis natto. Int. J. Biol. Macromol. 2021, 192, 684–691. [Google Scholar] [CrossRef]
- de Araújo Etchepare, M.; Raddatz, G.C.; de Moraes Flores, É.M.; Zepka, L.Q.; Jacob-Lopes, E.; Barin, J.S.; Ferreira Grosso, C.R.; de Menezes, C.R. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT 2016, 65, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Krunić, T.; Obradović, N.S.; Rakin, M.B. Application of whey protein and whey protein hydrolysate as protein based carrier for probiotic starter culture. Food Chem. 2019, 293, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Krunić, T.Z.; Rakin, M.B. Enriching alginate matrix used for probiotic encapsulation with whey protein concentrate or its trypsin-derived hydrolysate: Impact on antioxidant capacity and stability of fermented whey-based beverages. Food Chem. 2022, 370, 130931. [Google Scholar] [CrossRef] [PubMed]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.S.; Anandharamakrishnan, C. Effect of whey protein—Alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- Dehkordi, S.S.; Alemzadeh, I.; Vaziri, A.S.; Vossoughi, A. Optimization of Alginate-Whey Protein Isolate Microcapsules for Survivability and Release Behavior of Probiotic Bacteria. Appl. Biochem. Biotechnol. 2020, 190, 182–196. [Google Scholar] [CrossRef]
- Nasiri, H.; Golestan, L.; Shahidi, S.-A.; Darjani, P. Encapsulation of Lactobacillus casei in sodium alginate microcapsules: Improvement of the bacterial viability under simulated gastrointestinal conditions using wild sage seed mucilage. J. Food Meas. Charact. 2021, 15, 4726–4734. [Google Scholar] [CrossRef]
- Pitigraisorn, P.; Srichaisupakit, K.; Wongpadungkiat, N.; Wongsasulak, S. Encapsulation of Lactobacillus acidophilus in moist-heat-resistant multilayered microcapsules. J. Food Eng. 2017, 192, 11–18. [Google Scholar] [CrossRef]
- Mei, L.; He, F.; Zhou, R.-Q.; Wu, C.-D.; Liang, R.; Xie, R.; Ju, X.-J.; Wang, W.; Chu, L.-Y. Novel Intestinal-Targeted Ca-Alginate-Based Carrier for pH-Responsive Protection and Release of Lactic Acid Bacteria. ACS Appl. Mater. Interfaces 2014, 6, 5962–5970. [Google Scholar] [CrossRef]
- Lv, X.J.; Liu, Y.E.; Song, S.B.; Tong, C.C.; Shi, X.Y.; Zhao, Y.; Zhang, J.S.; Hou, M.X. Influence of chitosan oligosaccharide on the gelling and wound healing properties of injectable hydrogels based on carboxymethyl chitosan/alginate polyelectrolyte complexes. Carbohydr. Polym. 2019, 205, 312–321. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef]
- Yilmaz, B.; Juillerat, P.; Oyas, O.; Ramon, C.; Bravo, F.D.; Franc, Y.; Fournier, N.; Michetti, P.; Mueller, C.; Geuking, M.; et al. Microbial network disturbances in relapsing refractory Crohn’s disease (Vol 25, p. 323, 2019). Nat. Med. 2019, 25, 701. [Google Scholar] [CrossRef] [Green Version]
- Shamoon, M.; Martin, N.M.; O’Brien, C.L. Recent advances in gut Microbiota mediated therapeutic targets in inflammatory bowel diseases: Emerging modalities for future pharmacological implications. Pharmacol. Res. 2019, 148, 104344. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Lv, L.X.; Fang, D.Q.; Wu, W.R.; Hu, C.X.; Xu, L.C.; Chen, Y.F.; Guo, J.; Hu, X.J.; Li, A.; et al. Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 prevents CCl4-induced liver cirrhosis by protecting the intestinal barrier in rats. Sci. Rep. 2017, 7, 6927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swamy, B.Y.; Yun, Y.-S. In vitro release of metformin from iron (III) cross-linked alginate–carboxymethyl cellulose hydrogel beads. Int. J. Biol. Macromol. 2015, 77, 114–119. [Google Scholar] [CrossRef]
- Fontes, G.C.; Calado, V.M.A.; Rossi, A.M.; Da Rocha-Leão, M.H.M. Characterization of Antibiotic-Loaded Alginate-Osa Starch Microbeads Produced by Ionotropic Pregelation. BioMed Res. Int. 2013, 2013, 472626. [Google Scholar] [CrossRef]
- Adamczak, M.I.; Hagesaether, E.; Smistad, G.; Hiorth, M. An in vitro study of mucoadhesion and biocompatibility of polymer coated liposomes on HT29-MTX mucus-producing cells. Int. J. Pharm. 2016, 498, 225–233. [Google Scholar] [CrossRef]
- Popeski-Dimovski, R. Work of adhesion between mucin macromolecule and calcium-alginate gels on molecular level. Carbohydr. Polym. 2015, 123, 146–149. [Google Scholar] [CrossRef]
- Peres, L.B.; dos Anjos, R.S.; Tappertzhofen, L.C.; Feuser, P.E.; de Araujo, P.H.H.; Landfester, K.; Sayer, C.; Muñoz-Espí, R. pH-responsive physically and chemically cross-linked glutamic-acid-based hydrogels and nanogels. Eur. Polym. J. 2018, 101, 341–349. [Google Scholar] [CrossRef]
- Ruan, C.-Q.; Strømme, M.; Lindh, J. Preparation of porous 2,3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye. Carbohydr. Polym. 2018, 181, 200–207. [Google Scholar] [CrossRef]
- Kim, U.-J.; Kim, H.J.; Choi, J.W.; Kimura, S.; Wada, M. Cellulose-chitosan beads crosslinked by dialdehyde cellulose. Cellulose 2017, 24, 5517–5528. [Google Scholar] [CrossRef]
- Chalitangkoon, J.; Wongkittisin, M.; Monvisade, P. Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int. J. Biol. Macromol. 2020, 159, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, H.M.S.; Riaz, A.; Hamed, Y.S.; Abdin, M.; Chen, G.J.; Wan, P.; Zeng, X.X. Production and characterization of CMC-based antioxidant and antimicrobial films enriched with chickpea hull polysaccharides. Int. J. Biol. Macromol. 2018, 118, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Medronho, B.; Alves, L.; Da Silva, G.J.; Miguel, M.G.; Lindman, B. Development of carboxymethyl cellulose-chitosan hybrid micro- and macroparticles for encapsulation of probiotic bacteria. Carbohydr. Polym. 2017, 175, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Narayana, S.N.G.H.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Krunic, T.; Rakin, M.; Bulatovic, M.; Zaric, D. The Contribution of Bioactive Peptides of Whey to Quality of Food Products; Elsevier Science: Amsterdam, The Nederland, 2018; Volume 18, pp. 251–285. [Google Scholar]
- Mann, B.; Kumari, A.; Kumar, R.; Sharma, R.; Prajapati, K.; Mahboob, S.; Athira, S. Antioxidant activity of whey protein hydrolysates in milk beverage system. J. Food Sci. Technol. 2014, 52, 3235–3241. [Google Scholar] [CrossRef]
- Ramos, O.L.; Pereira, R.N.; Rodrigues, R.; Teixeira, J.A.; Vicente, A.A.; Malcata, F.X. Physical effects upon whey protein aggregation for nano-coating production. Food Res. Int. 2014, 66, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Ben Messaoud, G.; Sanchez-Gonzalez, L.; Jacquot, A.; Probst, L.; Desobry, S. Alginate/sodium caseinate aqueous-core capsules: A pH-responsive matrix. J. Colloid Interf. Sci. 2015, 440, 1–8. [Google Scholar] [CrossRef]
- Léonard, L.; Beji, O.; Arnould, C.; Noirot, E.; Bonnotte, A.; Gharsallaoui, A.; Degraeve, P.; Lherminier, J.; Saurel, R.; Oulahal, N. Preservation of viability and anti-Listeria activity of lactic acid bacteria, Lactococcus lactis and Lactobacillus paracasei, entrapped in gelling matrices of alginate or alginate/caseinate. Food Control 2015, 47, 7–19. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zhang, T.; Liu, T.; Yang, J.; Luo, X.; Li, H.; Xue, C.; Yu, J. Co-encapsulation of Lactobacillus paracasei with lactitol in caseinate gelation cross-linked by Zea mays transglutaminase. LWT 2021, 147, 111535. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Li, X.-Y.; Liu, B.-J.; Meng, X.-H. Microencapsulation of Lactobacillus bulgaricus and survival assays under simulated gastrointestinal conditions. J. Funct. Foods 2017, 29, 248–255. [Google Scholar] [CrossRef]
- Anal, A.K.; Tobiassen, A.; Flanagan, J.; Singh, H. Preparation and characterization of nanoparticles formed by chitosan–caseinate interactions. Colloids Surf. B: Biointerfaces 2008, 64, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Prebiotics: The Concept Revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Sada, A.; Orlando, P. Fermentative ability of alginate-prebiotic encapsulated Lactobacillus acidophilus and survival under simulated gastrointestinal conditions. J. Funct. Foods 2009, 1, 319–323. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Cui, S.W.; Guo, Q.B.; Ding, H.H. Some physicochemical properties of sage (Salvia macrosiphon) seed gum. Food Hydrocoll. 2014, 35, 453–462. [Google Scholar] [CrossRef]
- Rohman, S.; Kaewtatip, K.; Kantachote, D.; Tantirungkij, M. Encapsulation of Rhodopseudomonas palustris KTSSR54 using beads from alginate/starch blends. J. Appl. Polym. Sci. 2020, 138, 50084. [Google Scholar] [CrossRef]
- Martin, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716. LWT 2013, 53, 480–486. [Google Scholar] [CrossRef]
- Chan, E.-S.; Wong, S.-L.; Lee, P.-P.; Lee, J.S.; Ti, T.B.; Zhang, Z.B.; Poncelet, D.; Ravindra, P.; Phan, S.-H.; Yim, Z.-H. Effects of starch filler on the physical properties of lyophilized calcium–alginate beads and the viability of encapsulated cells. Carbohydr. Polym. 2011, 83, 225–232. [Google Scholar] [CrossRef]
- Atraki, R.; Azizkhani, M. Survival of probiotic bacteria nanoencapsulated within biopolymers in a simulated gastrointestinal model. Innov. Food Sci. Emerg. Technol. 2021, 72, 102750. [Google Scholar] [CrossRef]
- Ghorbani, S.; Maryam, A. Encapsulation of lactic acid bacteria and Bifidobacteria using starch-sodium alginate nanofibers to enhance viability in food model. J. Food Process. Preserv. 2021, 45, e16048. [Google Scholar] [CrossRef]
- Etchepare, M.D.A.; Raddatz, G.C.; Cichoski, A.J.; Flores, E.M.M.; Barin, J.S.; Zepka, L.Q.; Jacob-Lopes, E.; Grosso, C.R.F.; de Menezes, C.R. Effect of resistant starch (Hi-maize) on the survival of Lactobacillus acidophilus microencapsulated with sodium alginate. J. Funct. Foods 2016, 21, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Li, J.; Song, R.; Zhou, B.; Li, B.; Liang, H. Carboxymethylpachymaran/alginate gel entrapping of natural pollen capsules for the encapsulation, protection and delivery of probiotics with enhanced viability. Food Hydrocoll. 2021, 120, 106855. [Google Scholar] [CrossRef]
- Hadidi, M.; Majidiyan, N.; Jelyani, A.Z.; Moreno, A.; Hadian, Z.; Khanegah, A.M. Alginate/Fish Gelatin-Encapsulated Lactobacillus acidophilus: A Study on Viability and Technological Quality of Bread during Baking and Storage. Foods 2021, 10, 2215. [Google Scholar] [CrossRef] [PubMed]
- Grzywaczyk, A.; Zdarta, A.; Jankowska, K.; Biadasz, A.; Zdarta, J.; Jesionowski, T.; Kaczorek, E.; Smułek, W. New Biocomposite Electrospun Fiber/Alginate Hydrogel for Probiotic Bacteria Immobilization. Materials 2021, 14, 3861. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, Z.; Meral, R.; Karakaş, C.Y.; Dertli, E.; Yilmaz, M.T. A novel strategy for probiotic bacteria: Ensuring microbial stability of fish fillets using characterized probiotic bacteria-loaded nanofibers. Innov. Food Sci. Emerg. 2018, 48, 212–218. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Taylan, O.; Karakas, C.Y.; Dertli, E. An alternative way to encapsulate probiotics within electrospun alginate nanofibers as monitored under simulated gastrointestinal conditions and in kefir. Carbohydr. Polym. 2020, 244, 116447. [Google Scholar] [CrossRef]
- Chang, Y.; Li, J.J.; Sun, T.S.; Zhou, X.D. Grafting starch nanocrystals onto the surface of sisal fibers and consequent improvement of interfacial adhesion in sisal reinforced starch composite. J. Appl. Polym. Sci. 2019, 136, 47202. [Google Scholar] [CrossRef]
- Przyklenk, M.; Vemmer, M.; Hanitzsch, M.; Patel, A. A bioencapsulation and drying method increases shelf life and efficacy ofMetarhizium brunneumconidia. J. Microencapsul. 2017, 34, 498–512. [Google Scholar] [CrossRef]
- Khlibsuwan, R.; Tansena, W.; Pongjanyakul, T. Modification of alginate beads using gelatinized and ungelatinized arrowroot (Tacca leontopetaloides L. Kuntze) starch for drug delivery. Int. J. Biol. Macromol. 2018, 118, 683–692. [Google Scholar] [CrossRef]
- He, Y.H.; Wu, Z.S.; Tu, L.; Han, Y.J.; Zhang, G.L.; Li, C. Encapsulation and characterization of slow-release microbial fertilizer from the composites of bentonite and alginate. Appl. Clay Sci. 2015, 109–110, 68–75. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Brinques, G.B.; Ayub, M.A.Z. Probiotics production and alternative encapsulation methodologies to improve their viabilities under adverse environmental conditions. Int. J. Food Sci. Nutr. 2016, 67, 929–943. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.; Millon, C.; Nuñez, H.; Pinto, M.; Valencia, P.; Acevedo, C.; Simpson, R. Study of effect of sodium alginate on potato starch digestibility during in vitro digestion. Food Hydrocoll. 2015, 44, 328–332. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Liu, C.-M.; Gong, J. Issues deserve attention in encapsulating probiotics: Critical review of existing literature. Crit. Rev. Food Sci. Nutr. 2017, 57, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Nazhand, A.; Lucarini, M.; Atanasov, A.G.; Souto, E.B.; Novellino, E.; Capasso, R.; Santini, A. An Updated Overview on Nanonutraceuticals: Focus on Nanoprebiotics and Nanoprobiotics. Int. J. Mol. Sci. 2020, 21, 2285. [Google Scholar] [CrossRef] [Green Version]
- Zupančič, S.; Škrlec, K.; Kocbek, P.; Kristl, J.; Berlec, A. Effects of Electrospinning on the Viability of Ten Species of Lactic Acid Bacteria in Poly(Ethylene Oxide) Nanofibers. Pharmaceutics 2019, 11, 483. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimnejad, P.; Khavarpour, M.; Khalili, S. Survival of Lactobacillus Acidophilus as Probiotic Bacteria using Chitosan Nanoparticles. Int. J. Eng. 2017, 30, 456–463. [Google Scholar] [CrossRef]
- Hu, C.; Gong, R.H.; Zhou, F. Electrospun Sodium Alginate/Polyethylene Oxide Fibers and Nanocoated Yarns. Int. J. Polym. Sci. 2015, 2015, 126041. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Ismail, I.H.; Balloch, A.; Mui, M.; Hoe, E.; Lamb, K.; Tang, M.L.K. Maternal Supplementation with LGG Reduces Vaccine-Specific Immune Responses in Infants at High-Risk of Developing Allergic Disease. Front. Immunol. 2013, 4, 381. [Google Scholar] [CrossRef] [Green Version]
- de López Lacey, A.M.; López-Caballero, M.E.; Montero, P. Agar films containing green tea extract and probiotic bacteria for extending fish shelf-life. LWT 2014, 55, 559–564. [Google Scholar] [CrossRef]
- Kriegel, C.; Kit, K.M.; McClements, D.; Weiss, J. Nanofibers as Carrier Systems for Antimicrobial Microemulsions. Part I: Fabrication and Characterization. Langmuir 2008, 25, 1154–1161. [Google Scholar] [CrossRef]
- Jayakumar, R.; Rani, V.V.D.; Shalumon, K.T.; Kumar, P.T.S.; Nair, S.V.; Furuike, T.; Tamura, H. Bioactive and osteoblast cell attachment studies of novel α- and β-chitin membranes for tissue-engineering applications. Int. J. Biol. Macromol. 2009, 45, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Patel, S.; Kim, S.-K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Dianawati, D.; Mishra, V.; Shah, N.P. Survival of Microencapsulated Probiotic Bacteria after Processing and during Storage: A Review (Vol. 56, p. 1685, 2016). Crit. Rev. Food Sci. Nutr. 2016, 56, 2250. [Google Scholar]
- Li, W.; Liu, L.M.; Tian, H.F.; Luo, X.G.; Liu, S.L. Encapsulation of Lactobacillus plantarum in cellulose based microgel with controlled release behavior and increased long-term storage stability. Carbohydr. Polym. 2019, 223, 115065. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Diego-Taboada, A.; Maillet, L.; Banoub, J.H.; Lorch, M.; Rigby, A.S.; Boa, A.N.; Atkin, S.L.; Mackenzie, G. Protein free microcapsules obtained from plant spores as a model for drug delivery: Ibuprofen encapsulation, release and taste masking. J. Mater. Chem. B 2013, 1, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.F.; Hwang, Y.; Ibrahim, M.S.; Ferracci, G.; Cho, N.J. Influence of Chemical and Physical Change of Pollen Microgels on Swelling/De-Swelling Behavior. Macromol. Rapid Comm. 2020, 41, 2000155. [Google Scholar] [CrossRef]
- Potroz, M.G.; Mundargi, R.C.; Gillissen, J.J.; Tan, E.L.; Meker, S.; Park, J.H.; Jung, H.; Park, S.; Cho, D.; Bang, S.I.; et al. Plant-Based Hollow Microcapsules for Oral Delivery Applications: Toward Optimized Loading and Controlled Release. Adv. Funct. Mater. 2017, 27, 1700270. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Pei, Y.Q.; Wang, S.S.; Zhou, B.; Hou, X.Y.; Li, J.; Li, B.; Liang, H.S. Designable Carboxymethylpachymaran/Metal Ion Architecture on Sunflower Sporopollenin Exine Capsules as Delivery Vehicles for Bioactive Macromolecules. J. Agric. Food Chem. 2020, 68, 13990–14000. [Google Scholar] [CrossRef]
- Iqbal, U.H.; Westfall, S.; Prakash, S. Novel microencapsulated probiotic blend for use in metabolic syndrome: Design and in-vivo analysis. Artif. Cells Nanomed. Biotechnol. 2018, 46, S116–S124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Ji, Y.R.; Lee, S.; Choi, M.-J.; Cho, Y. Microencapsulation of Probiotic Lactobacillus acidophilus KBL409 by Extrusion Technology to Enhance Survival under Simulated Intestinal and Freeze-Drying Conditions. J. Microbiol. Biotechnol. 2019, 29, 721–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meurman, J.H.; Stamatova, I.V. Probiotics: Evidence of Oral Health Implications. Folia Medica 2018, 60, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Mirtič, J.; Rijavec, T.; Zupančič, S.; Pobirk, A.Z.; Lapanje, A.; Kristl, J. Development of probiotic-loaded microcapsules for local delivery: Physical properties, cell release and growth. Eur. J. Pharm. Sci. 2018, 121, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Brachkova, M.; Marques, P.; Rocha, J.; Sepodes, B.; Duarte, M.; Pinto, J. Alginate films containing Lactobacillus plantarum as wound dressing for prevention of burn infection. J. Hosp. Infect. 2011, 79, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Mirzamani, S.S.; Bassiri, A.R.; Tavakolipour, H.; Azizi, M.H.; Kargozari, M. Survival of fluidized bed encapsulated Lactobacillus acidophilus under simulated gastro-intestinal conditions and heat treatment during bread baking. J. Food Meas. Charact. 2021, 15, 5477–5484. [Google Scholar] [CrossRef]
- Soukoulis, C.; Yonekura, L.; Gan, H.-H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef]
- Yao, M.F.; Li, B.; Ye, H.W.; Huang, W.H.; Luo, Q.X.; Xiao, H.; McClements, D.J.; Li, L.J. Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles. Food Hydrocoll. 2018, 83, 246–252. [Google Scholar] [CrossRef]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; et al. Can dynamic in vitro digestion systems mimic the physiological reality? Crit. Rev. Food Sci. Nutr. 2019, 59, 1546–1562. [Google Scholar] [CrossRef] [Green Version]
- Tamargo, A.; Gil-Sanchez, I.; Miralles, B.; Martin, D.; Garcia-Risco, M.R.; Fornari, T.; Bartolome, B.; Moreno-Arribas, M.V.; Cueva, C. The dynamic gastrointestinal simulator (simgi (R)): A useful tool for clinical nutrition. Nutr. Hosp. 2017, 34, 1489–1496. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Nanomaterial-based encapsulation for controlled gastrointestinal delivery of viable probiotic bacteria. Nanoscale Adv. 2021, 3, 2699–2709. [Google Scholar] [CrossRef]






| Encapsulated Strain | Application | Remarks | References |
|---|---|---|---|
| Saccharomyces cerevisiae strains | -- | Encapsulation yield was at least 60% (100% for some strains) and yeasts survived in beads for 30 days at 4 °C. | [25] |
| Saccharomyces boulardii and Enterococcus faecium | -- | Higher survival rate of S. burrici and E. faecium (increased by 25% and 40%) at high temperature and high humidity. Higher survival rate in the SGF 1 (increased by 60% and 25%) and SIF 2 (increased by 15% and 20%). | [40] |
| Fruit-derived lactic acid bacteria (LAB) | Reduce the anthracnose lesion development in guava and mango. | Sodium alginate coatings loaded with laboratory strains had higher ALDR% (anthracnose lesion diameter reduction) values in guava and mango. | [41] |
| Bacillus licheniformis | -- | Cell survival rate for 30 days at 4 °C was 55.58 ± 2.35%. Higher survival rate of alginate-encapsulated bacteria (100.0 ± 7.72%, 62.71 ± 4.81%, 80.79 ± 7.40%, and 51.29 ± 0.42%, respectively) in the simulated shrimp digestive tract (stomach, hepatopancreas, and midgut or hindgut). | [42] |
| CoatingMaterial | Encapsulated Strain | Application | Remarks | References |
| Alg + CS | Ligilactobacillus salivarius Li01 (Li01) | Treatment of inflammatory bowel disease. | After 2 h incubation in the digestive solution, the cell number of probiotics remained above 6 log CFU/mL. | [55] |
| Alg + CS | Bacillus coagulans (BC) | Treatment of colitis and abdominal pain associated with irritable bowel syndrome. | Less than 1 log reduction in CFU was observed in SGF conditions and less than 2 log reduction in CFU was observed in 4% bile salts after 2 h. Adhesion of encapsulated BC is nearly 1.5 times higher than that of ordinary BC. | [56] |
| Alg + CS | Lactoferm ABY 6 1 | Prepare Greek yogurt. | The survival of bacteria in the simulated gastrointestinal environment was significantly improved. | [57] |
| Alg + CS | Lactobacillus plantarum KCC-42 | Against different pathogenic fungal strains such as A. fumigatus, A. clavatus. | Higher viability rate (7.48 × 105 CFU/mL) and tolerance to high acidic/pancreatin medium compared in mock gastrointestinal fluids. | [58] |
| Alg + CS | Bifidobacterium longum strain DD98 | Regulating intestinal flora, increasing immune function, improving lipid metabolism. | Temperature and pH stability are significantly improved. Higher viability of encapsulated B. longum (reduced by 1.27 log CFU at 120 min at pH 2.5). Higher viability of encapsulated B. longum (decreased by 2.68 log CFU at 120 min when exposed to intestinal fluid with bile salt (1%)). | [59] |
| Alg + CS | Lactobacillus gasseri Bifidobacterium bifidum | Prevent gastrointestinal diseases. | Cell survival after exposure to SGF for 5 min was 95% of the initial population found in microencapsulated bacteria. Survival after incubating at SIF for 120 min, 98.86% and 96.72%. | [60] |
| Alg + CS + carboxymethyl cellulose (CMC) | Bacillus subtilis natto | Protect and promote the growth of probiotics. | Higher embedding rate (67.3%). Sustained release lasted for more than 10 h. Shelf life of viable Bacillus subtilis natto lasted for up to 20 days. | [61] |
| Alg + Hi maize + CS | Lactobacillus acidophilus | -- | Longer survival at room temperature (6 months), freezing temperature (135 days), and cold storage temperature (105 days). | [62] |
| Alg + WPC 2/WPH 3 | Lactoferm ABY 6 | Prepare Greek yogurt. | More cells survived (more than 96%) after 4 h of gastrointestinal tract simulation compared with free culture cell (25.67%). | [63] |
| Alg + WPC/WPH | Lactoferm ABY 6 | Prepare Greek yogurt. | High efficiency of encapsulation (between 92.98 and 94.20%). | [64] |
| Alg + WPI 4/DWPI 5 | Lactobacillus plantarum (mtcc 5422) | Improve the intestinal microenvironment. | DWPI + Alg improved probiotics survival (96% by freeze-drying and 87% by spray-drying). | [65] |
| Alg + WPI | Lactobacillus acidophilus | -- | By increasing the concentration of WPI, efficiency of encapsulation was significantly increased to 81.42–97.51%. | [66] |
| Alg + WSSM 6 | Lactobacillus casei | -- | The log reduction of encapsulated bacteria after 120 min incubation in SGF and SIF was 3.58–4.52 compared to 6.53 for free cells. | [67] |
| Alg + acidified egg albumen (EA) + stearic acid (SA) + cassava starch | Lactobacillus acidophilus | -- | Less reduction of EA–SA-coated cells wrapped in microcapsules (1.3~0.6 log CFU/g) compared with free cells by exposure to moist heat at 70 °C for 30 min. | [68] |
| Alg + protamine | Lactobacillus casei | Improve intestinal flora, enhance immunity, inhibit tumor growth. | Survival 60 times higher in SGF compared to free cells. The speed of whole release process of encapsulated L. casei in pH 7.0 SIF is 7.6 times faster than the control group. | [69] |
| Coating Material | Encapsulated Strain | Application | Remarks | References |
|---|---|---|---|---|
| Alg + cassava starch | Rhodopseudomonas palustris KTSSR54 | Promote plant growth. | The encapsulation efficiency with alginate alone was 50.56%, compared to 70.83% when the starch content was 4% (w/v). | [99] |
| Alg + corn starch | Lactobacillus fermentum CECT5716 | -- | Encapsulation efficiency of starch particles increased significantly (from 74.41% to 97.26%). | [100] |
| Alg + native corn starch | Lactobacillus casei 01 | -- | Beads with a starch concentration of 600 g/L had 4 times more storage stability than without starch and had a porosity rate of 1/3. After lyophilization, the survival of cells encapsulated within the beads with starch was 100 times higher than that of the control group. | [101] |
| Alg + corn starch | Lactobacillus acidophilus (LA5), Lactobacillus rhamnosus 23,527 LGG, Bifidobacterium bifidum, Bifidobacterium animalis | -- | The viability rate of lactobacilli and bifidobacteria, after 120 min, in gastric condition obtained 90% and 84.1% and in intestinal condition 71.7% and 77.8% of the initial count compared to encapsulated control, respectively, (p < 0.01). | [102] |
| Alg + corn starch | Lactobacillus rhamnosus 23,527 LGG, Lactobacillus acidophilus (LA5), Bifidobacterium bifidum, Bifidobacterium animalis | -- | Temperature stability is significantly improved. The survival rates of nanoencapsulated LAB and Bifidobacterium increased from 87.7% to 97.9% and from 86.3% to 96.9%, respectively, after 20 days of yogurt preservation. | [103] |
| Alg + Hi-maize | Lactobacillus acidophilus | -- | The freeze-dried microparticles of alginate and alginate + Hi-maize had diameters of 114.51 and 78.49 µm, respectively. | [104] |
| Alg + carboxymethylpachymaran (CMP) | Lactobacillus plantarum | -- | The composite coating showed excellent performance in terms of sustainable release and freeze-drying stability: 107 CFU/mL before and after freeze-drying. Composite coating has good storage stability: 2.09 × 106 CFU/mL at 4 °C for 90 days. | [105] |
| Alg + fish gelatin | Lactobacillus acidophilus LA-5 | -- | Compared with the control (free bacteria), the activity of encapsulated L. acidophilus increased to 2.49 and 3.07 log CFU/g during baking and storage, respectively. Good storage stability: 106 CFU/g after 4 days. | [106] |
| Alg + polystyrene (PS) | Lactobacillus plantarum 2675 | -- | Temperature and UV light had low inhibitory effects on the growth of immobilized cells, from 81% to 40% and from 64% to 48%, respectively. | [107] |
| Alg + Poly(vinyl alcohol)(PVA) | Lactobacillus rhamnosus GG | Treatment of allergic diseases | Zeta potential values for VS 1 and VSPBe 2 were −6.29 mV and −7.74 mV, respectively. The bacterial survival rate was up to 9.62 log CFU/mL 11% (1st day), 37% (3rd day), 29% (5th day), and 8% (9th day) decrement in TMAB 3 counts of coated fish fillets compared to uncoated fish fillets (p < 0.05). | [108] |
| Alg + PVA | Lactobacillus paracasei KS-199 | -- | Enhanced protection ability of the mats was observed in thermal degradation assays (weight loss from 93.4 to 84.5%). Increased survival of strains in simulated gastric juice (the viability rate from 64.1 to 70.8 log CFU/mL). | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Gao, S.; Yun, S.; Zhang, M.; Peng, L.; Li, Y.; Zhou, Y. Microencapsulating Alginate-Based Polymers for Probiotics Delivery Systems and Their Application. Pharmaceuticals 2022, 15, 644. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15050644
Wang X, Gao S, Yun S, Zhang M, Peng L, Li Y, Zhou Y. Microencapsulating Alginate-Based Polymers for Probiotics Delivery Systems and Their Application. Pharmaceuticals. 2022; 15(5):644. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15050644
Chicago/Turabian StyleWang, Xiaochen, Shukun Gao, Shuaiting Yun, Mingjing Zhang, Liyang Peng, Yingxiu Li, and Yanxia Zhou. 2022. "Microencapsulating Alginate-Based Polymers for Probiotics Delivery Systems and Their Application" Pharmaceuticals 15, no. 5: 644. https://0-doi-org.brum.beds.ac.uk/10.3390/ph15050644