1. Introduction
Stroke is the third and fourth leading cause of annual deaths in Korea [
1] and the United States [
2,
3], respectively. Worldwide, stroke is the leading cause of permanent disability in adults [
3,
4]. Approximately 90% of stroke cases are cerebral infarctions caused by thrombus or embolization [
2,
3,
5,
6]. Further, its incidence is increasing, given the increasingly aging society; accordingly, urgent measures are required from a health–economic perspective.
Tissue plasminogen activator (tPA), which is a thrombolytic agent, is currently the only drug approved by the U.S. Food and Drug Administration for treating cerebral infarction, and should be administered within 3 h of the onset of acute cerebral infarction [
7]. However, tPA is clinically used only in limited cases (4–7%) of patients with acute cerebral infarction, given the short therapeutic window, risk of bleeding, and nonresponsiveness in some patients [
3,
8]. Numerous studies have attempted to develop neuroprotection drugs for cerebral infarction; however, there have been no successful clinical trials [
9]. To overcome these challenges, there are currently many studies on new treatment methods being conducted [
3,
6,
9].
There has been increasing attention on traditional Korean medicine, which applies natural products, as a complementary or alternative treatment for cerebral infarction [
10,
11,
12,
13]. Recently, Kim et al. [
14] developed a drug comprising Coptidis Rhizoma, Scutellariae Radix, Phellodendri Cortex, Gardeniae Fructus, and Rhei Radix et Rhizoma extracted with 80% ethanol and named it Chunghyuldan (CD), which has been extensively studied [
13,
14,
15,
16,
17,
18,
19,
20,
21]. Specifically, a study using an animal model of cerebral infarction demonstrated the neuroprotective effect of CD [
21]. Additionally, a clinical study on patients with small-vessel cerebral infarction showed that CD exerted a significant inhibitory effect on cerebral infarction recurrence, compared with antiplatelet treatment [
13,
22,
23].
Numerous studies have investigated the treatment of ischemic heart disease using cardiotonic pills, which comprise Salviae Miltiorrhizae Radix, Notoginseng Radix et Rhizoma, and Bomeolum [
24,
25,
26]; further, cardiotonic pills have been shown to exert neuroprotective effects [
27,
28,
29]. Based on this evidence, in anticipation of the enhancement of the neuroprotective effect when CD and cardiotonic pills are used together, Kyung Hee University Korean Medicine Hospital Stroke and Brain Diseases Center developed Geopung-Chunghyuldan (GCD) by combining CD with cardiotonic pills. However, the neuroprotective effect of GCD and the difference in efficacy from CD have not been elucidated, to date. Therefore, studies should investigate whether combining CD and the herbal material used in cardiotonic pills could exert a synergistic effect that could enhance the therapeutic efficacy against cerebral infarction.
We aimed to evaluate the neuroprotective effect of Geopung-Chunghyuldan (GCD) combined with Salviae Miltiorrhizae Radix, Notoginseng Radix et Rhizoma, and Bomeolum using in vitro and in vivo stroke models.
3. Discussion
This study evaluated the neuroprotective effect of GCD in acute cerebral infarction using both in vitro and in vivo models. We found that GCD showed a significant neuroprotective effect in both models, indicating an improved synergistic neuroprotective effect compared with conventional CD.
Experimental models for studying cerebral infarction can be mainly divided into in vitro and animal models [
6]. In vitro models typically apply glucose deprivation (GD) or OGD. The GD method involves neuronal culturing in a glucose-free medium. Contrastingly, the OGD method combines the GD method with hypoxic exposure and is widely used, since it is relatively more similar to the method for in vivo cerebral infarction [
6,
30,
31,
32]. Animal models for cerebral infarction are mainly classified into the global and focal ischemic stroke models. The global ischemic stroke model can either involve four-vessel or two-vessel occlusion, depending on the occlusive blood vessel; further, it clinically mimics hypoxic brain injury due to cardiac arrest. The focal ischemic stroke model involves transient, permanent, or photothrombotic distal middle cerebral artery occlusion; further, it mimics cerebral infarction in humans. The transient method, which temporarily occludes and recanalizes the middle cerebral artery, and the pMCAO method, which permanently occludes the middle cerebral artery by cauterizing or binding with a thread, are widely used depending on the study purpose [
6,
31,
33,
34,
35]. The present study used the aforementioned OGD and pMCAO models, which are relatively widely used in studies on cerebral infarction.
Previous studies have shown that CD (Sample A of this study) exerts antihypertensive [
18,
20], antihyperlipidemic [
14,
16], anti-inflammatory [
17,
19], and antiapoptotic effects on vascular endothelial cells [
15]. Additionally, numerous studies have demonstrated the neuroprotective effects of CD. Ko et al. [
36] reported that CD exerted neuroprotective effects by reducing the expression of Bax, a proapoptotic protein, in neuroblastoma 2a cells in a mouse model established through hypoxia–reoxygenation. Kim et al. [
37] reported that CD exerted neuroprotective effects by inhibiting the production of reactive oxygen species (ROS) in in vitro and in vivo models of Parkinson′s disease. Nam et al. [
38] reported that CD exerted neuroprotective effects by reducing the production of nitric oxide, tumor necrosis factor-alpha, and prostaglandin E2 by activated microglia. Moreover, Cho et al. [
21] reported that CD ameliorated cerebral infarction in a tMCAO mouse model of focal cerebral infarction; further, this neuroprotective effect was attributed to the inhibition of microglial activation and neutrophil infiltration. Moreover, Cho et al. [
13] performed follow-up magnetic resonance imaging in patients with small-vessel cerebral infarction (SVD), and found that patients who took 600 mg of CD daily for 2 years had a significantly lower recurrence rate of cerebral infarction than patients who took antiplatelet drugs.
In our study, Sample A (CD) allowed a nonsignificant decrease in cerebral infarction, which is inconsistent with the results of a previous study using a rat tMCAO model [
21]. This could be attributed to differences between the pMCAO and tMCAO models. Accordingly, 30 mg/kg aspirin did not significantly reduce cerebral infarction, which is inconsistent with the previous report [
39,
40,
41]. In the pMCAO model, since the distal end of the middle cerebral artery is occluded, the resulting cerebral infarction size is relatively small compared with that in the tMCAO model, which involves occlusion of the proximal end. The lack of a significant difference in our study could be attributed to the small sample size.
Cardiotonic pills are used to treat angina, a coronary artery disease, and comprise Salviae Miltiorrhizae Radix, Notoginseng Radix et Rhizoma, and Bomeolum. Over 100 randomized controlled trials (RCTs) have been conducted in China to investigate cardiotonic pills. A systematic review of 60 RCTs reported that cardiotonic pills were more effective than isosorbide dinitrate in treating angina [
24]. In vitro studies have attributed the effects of cardiotonic pills to the lowering of endothelial cell–leukocyte adhesion by reducing endothelial cell adhesion molecules, including intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) [
42,
43], as well as the inhibition of platelet and leukocyte activation [
44]. Zhao et al. [
25] reported that cardiotonic pills could effectively prevent myocardial injury and microcirculation disorder after coronary artery ischemia/reperfusion in mice, which was attributed to antioxidant effects by Yang et al. [
26]. Additionally, in an animal model of hyperlipidemia, cardiotonic pills exerted antihyperlipidemic effects and improved erythrocyte deformability [
45]. Ling et al. [
46] reported that cardiotonic pills inhibited arteriosclerosis in a mouse model of high-fat diet-induced atherosclerosis, which was attributed to the inhibition of ICAM-1 expression. Another study described the inhibitory effects of cardiotonic pills on thrombogenesis [
47]. A clinical study on patients with hyperlipidemia reported that cardiotonic pills significantly decreased ICAM-1 and E-selectin expression [
48].
Although most studies on cardiotonic pills have focused on cardiovascular diseases, cardiotonic pills may be effective in cerebrovascular diseases, since they share the pathophysiological characteristic of arteriosclerosis with cardiovascular diseases. In our study, Sample B + C, which comprised the herbal materials present in cardiotonic pills, significantly reduced cerebral infarction. Therefore, cardiotonic pills may exert neuroprotective effects on cerebral infarction. Accordingly, Lee et al. [
27] reported that cardiotonic pills exerted neuroprotective effects against white matter and hippocampal damages induced by occlusion of both common carotid arteries in white mice, which was attributed to inhibition of the activation of inflammatory processes related to microglia and inflammatory mediators. Kwon et al. [
29] reported that cardiotonic pills exerted protective effects on neuroglia from oxidative damage, and increased regional cerebral blood flow in white mice. Xu et al. [
28] reported that cardiotonic pills reduced the recurrence rate of cerebral infarction/transient cerebral ischemia.
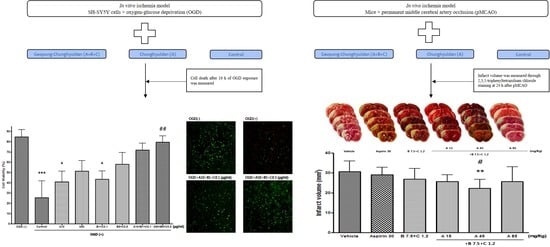
In the MTT assay, compared with the control group, Sample A and Sample B + C did not significantly increase the O.D. value at 48 h after OGD treatment; however, Sample A + B + C (at both the low [A10 + B1 + C0.1 (μg/mL)] and high [A50 + B5 + C0.5 (μg/mL] concentrations) significantly increased the O.D. value. Similar results regarding cell viability were observed in the cell count experiment. These findings indicate a synergistic neuroprotective effect of the Sample A + B + C complex compared with each sample.
Although the pathway by which GCD affected cell viability after OGD was not verified in this study, mitochondrial targeting is presumed to be the mechanism. Mitochondria are essential to maintaining cell energy homeostasis, and thus are inevitably associated with ischemic neuronal cell death [
49]. Mitochondria dysfunction is a major factor in ischemia/reperfusion injury that causes neuronal death [
50], and mitochondria targeting is being discussed as a neuroprotective strategy for the treatment and prevention of ischemic stroke [
51]. A previous study has shown that CD, part of GCD, has a neuroprotective effect by regulating mitochondrial dysfunction caused by ROS generation [
34]. Polyphenols, found in natural plants, affect mitochondrial biogenesis by modulating intracellular signaling pathways [
52]. The herbal medicines that make up GCD are all natural plants and contain polyphenols, supporting the mitochondrial targeting of GCD. However, this requires clarification through additional experiments.
Compared with the control group, the Sample A + B + C complex extract (fixed concentration of Sample A) and the Sample A + B + C complex extract (fixed Sample B + C concentration) nonsignificantly and significantly decreased the cerebral infarct volume, respectively. Since Sample A and Sample B + C alone did not significantly reduce the cerebral infarction volume, Sample A + B + C could be considered to have a synergistic neuroprotective effect, which is consistent with the results of the OGD experiment. Further, since Sample B + C, but not Sample A, reduced the cerebral infarction volume, Sample B + C could play a greater role in the observed synergistic effect than Sample A. Moreover, since the synergistic effect was observed at a specific mixing ratio, further studies are warranted to determine the optimal mixing ratio.
Although we did not elucidate the mechanism of action underlying the neuroprotective synergistic effects of GCD on acute cerebral infarction, it can be inferred from previous studies on CD and cardiotonic pills. CD and cardiotonic pills have been shown to treat ischemic brain injury through inflammation reduction by inhibiting microglia activation [
21,
27,
38]. Inflammatory processes contribute to acute brain injury after ischemia. Specifically, microglia can be activated to secrete various inflammatory substances and ROS, which further aggravates brain damage. Alternatively, circulating immune cells can be activated, with blood–brain barrier damage after cerebral infarction, promoting the expression of cell adhesion molecules (ICAM-1, VCAM-1) in vascular endothelial cells. As a result, various inflammatory substances are attached, allowing leukocytes to penetrate the brain parenchyma, which secondarily exacerbates inflammation and brain damage [
3]. Since the neuroprotective effect of CD could be attributed to the inhibition of leukocyte infiltration [
21], and cardiotonic pills can suppress ICAM-1 expression [
42,
43,
46,
48], the neuroprotective synergistic effects of GCD could be attributed to the reduction of inflammation through inhibition of the activation of circulating immune cells and microglia.
There has been increasing interest in collateral therapeutics for neuroprotection in cerebral infarction [
53,
54,
55]. Collateral therapeutics delay or prevent damage to the cranial nerves in the ischemic region by enhancing blood flow through the leptomeningeal collateral channel (leptomeningeal or pial collaterals). Currently, proposed approaches to this include increasing circulating blood flow, inducing hypertension, administering vasodilators, temporarily occluding the abdominal aorta, and electrically stimulating the parasympathetic ganglia [
54]. Since cardiotonic pills increase local cerebral blood flow [
29], the neuroprotective effect of GCD could have resulted from improved collateral circulation. Further studies are warranted to investigate the mechanism of action underlying the efficacy of GCD.
There are several significant aspects of this study. First, a novel alternative for the prevention and treatment of ischemic stroke was presented. CD, which is currently used for the treatment and prevention of ischemic stroke, has a recurrence rate of 6.2% for ischemic stroke due to small vessel disease over 5 years, which is known to be the highest among the previously announced stroke recurrence rates of antiplatelet agents [
23]. Since GCD is expected to have better ischemic stroke treatment and prevention effects according to the results of this study, it has potential as an alternative for existing antiplatelets. Second, the combination possibilities and advantages of several herbal medicines were suggested. Western medicines are often used in combination with medications with similar effects, such as dual antiplatelet therapy, to enhance the effect of antiplatelet agents. Considering that combining CD and cardiotonic pills, which have proven neuroprotective effects, leads to better neuroprotective effects of GCD, herbal medicines may have a synergistic effect when medicines with similar effects are combined. Collectively, these processes have opened up the possibility of various applications of herbal medicine as an alternative component for the treatment and prevention of ischemic stroke.
Despite the importance of our findings, future studies concerning physiological parameters of blood samples in the pMCAO model should be conducted. Since changes in the infarct volume in pMCAO may have been influenced by blood parameters at the time, it is necessary to measure physiological parameters by taking blood samples before and after pMCAO. In addition, the excellent neuroprotective effect of GCD was revealed, but the composition ratio of CD and three herbal medicines of cardiotonic pills, Salviae Miltiorrhizae Radix, Notoginseng Radix et Rhizoma, and Bomeolum, could not be concluded. It is essential to search for the optimal composition ratio for the homogenization of medications and maximization of efficacy. Finally, since the models that explored the neuroprotective effect of GCD in this study were in vitro and in vivo, additional verification through future clinical studies is required based on the evidence demonstrated here.