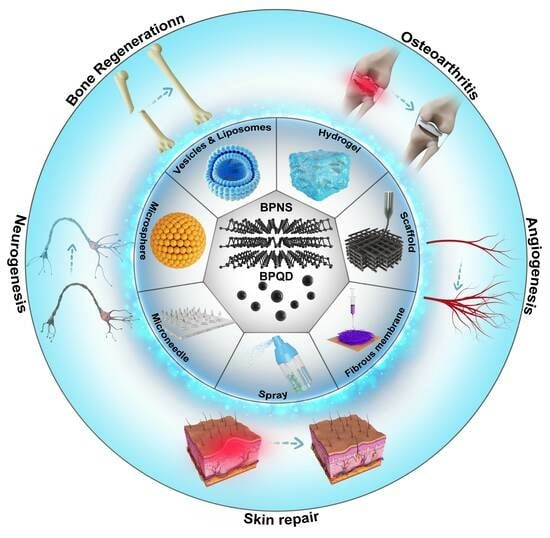
Advances in the Application of Black Phosphorus-Based Composite Biomedical Materials in the Field of Tissue Engineering
Abstract
:1. Introduction
2. The Physicochemical Properties and Biological Effects of Black Phosphorus Materials
2.1. Physical and Chemical Properties
2.1.1. Photothermal Effects
2.1.2. Photodynamic Effects
2.1.3. Drug Loading Performances
2.1.4. Electrical Conductivity
2.2. Biological Characteristics
2.2.1. Biocompatibility and Degradability
2.2.2. Osteoinduction
2.2.3. Antibacterial Properties
- (a)
- Physical damage caused by membrane damage. BP has an orthogonal crystal structure and fold surface, which can effectively make contact with the bacterial surface. The use of rough surfaces and sharp edges to destroy the bacterial membrane, causing bacterial death, is one of the mechanisms underlying the antimicrobial activity of BP, also known as the nanoknife mechanism [46]. BP exhibits time-and concentration-dependent antimicrobial activity against both Gram-negative and -positive bacteria, as the sharp edges of BPNS in the interaction with bacteria can cause physical damage to the bacterial membrane and RNA leakage, resulting in bacterial death [47].
- (b)
- ROS generation. Oxidative stress caused by ROS is the main mechanism of bacterial death, and ROS kills bacterial pathogens by destroying the cell membrane and bacterial intracellular molecules such as DNA, RNA, and protein interactions. Shaw demonstrated that the bactericidal properties of BP arise from its unique ability to produce ROS and have excellent antimicrobial effects against sensitive and resistant bacteria and even fungi [48]. BPNS can be used as an effective photosensitizer to produce 1O2 with a quantum yield of about 0.91. The bactericidal rate of BPNs to E. coli and B. subtilis reached 91.65% and 99.69%, proving that oxidative stress caused by ROS is one of the bactericidal mechanisms [46]. Tan et al. prepared antibiofilm containing BP and produced 1O2 under visible light to 99.3% and 99.2% [49].
- (c)
- The photothermal effect destroys the bacteria. The photothermal effect can inhibit bacterial growth and kill bacteria by altering bacterial cell membrane permeability and signal transmission pathways. BP has high photothermal conversion efficiency under near-infrared light sources [50]. Zhang et al. improved the stability of BP with quaternary chitosan, showing excellent photothermal capacity. The temperature of BP increased by nearly 30 °C after 10 min at 808 nm, and the sterilization rate against methicillin S. aureus and E. coli was greater than 95% at low doses [51].
2.2.4. Promotion of Neuronal Differentiation
2.3. Potential Clinical Applications
3. BP-Based Composite Biomaterials for Tissue Repair
3.1. BP-Based Hydrogels
3.1.1. Implantable BP-Based Hydrogels
3.1.2. Injectable BP-Based Hydrogels

3.1.3. BP-Based Hydrogel Dressings
3.1.4. BP-Based Spray Gel
| Materials | Modification | Property | Therapy Mode | Application | Ref. |
|---|---|---|---|---|---|
| BPNS | BP/PEA/GelMA hydrogel | Sustained supply of calcium-free phosphorus; Accelerate in situ mineralization deposition of osteocytes | hDPSCs; Rabbit model of cranial defects | bone regeneration | [44] |
| BPNS | BP/MgO/PVA/CS hydrogel | NIR photothermal antibacterial; Promote cell migration and osteogenic differentiation; | BMSCs; Rat model of cranial defects | bone regeneration | [63] |
| BPNS | BP/Gel hydrogel | NIR photothermal antibacterial; Eliminate the osteosarcoma cells; Enhance the bone regeneration capacity | hMSCs; Saos-2; Rat model of cranial defects | osteosarcoma; bone regeneration | [64] |
| BPNS | BP/DFO/Gel hydrogel | Superior swelling, degradation, and release rate; Promote neovascularization; Promote bone regeneration by activating the BMP/Runx 2 pathway; | BMSCs; Rat model of ischemic tibial defects; | ischemic bone defect | [65] |
| BPNS | BP/double network hydrogel | Promote cell proliferation and adhesion; Mechanical properties adjustable | hBMSCs; Rat model of cranial defects | bone regeneration | [66] |
| BPNS | BP/Mg double network hydrogel | Enhance early vascularization and neurogenesis; Promote bone regeneration and remodeling | HUVECs; NSCs; BMSCs; Rat model of bilateral skull defects | bone regeneration | [67] |
| BPNS | BP/CNT/OPF hydrogel | Conductive properties and syringeability; Enhance bone regeneration; Promote pro-osteoblast proliferation and differentiation | MC3T3-E1 cells; Rabbit model of Femur and spine defects | bone regeneration | [69] |
| BPNS | BP/CS/PRP hydrogel | Promote cell proliferation and adhesion; Protect the articular cartilage by reducing the friction; Generate ROS to suppress inflammation; Thermo responsive; | MSCs; Rat Model of rheumatoid arthritis. | rheumatoid arthritis | [72] |
| BPNS | BP/ZnO Gel hydrogel | Upregulate the expression of CD31 and α-SMA; Reduce inflammation and facilitate neovascularization | HUVECs; Rat model of full-thickness wound defect with bacterial infection | bacterial wound infections | [73] |
| BPQDs | BP/PVA/Alg hydrogel | NIR photothermal effect; ROS-generating and antibacterial; Reduce inflammatory response; Regulate the expression of VEGF and bFGF. | HUVECs; Rat model of diabetic wound infection | diabetic wound infections | [75] |
| BPQDs | BP/EGCG hydrogel | NIR photothermal effect; Generate ROS to suppress inflammation; Upregulate the expression of CD31 and bFGF; Promote wound healing by triggering the PI3K/AKT and ERK1/2 signaling pathways; Enhance cell proliferation and differentiation; | HUVECs; Rat model of diabetic burn-wound infection | diabetic wound infections | [76] |
| BPNS | BP/CS hydrogel | Generate 1O2 to suppress inflammation; Enhance the formation of the fibrinogen for accelerated incrustation; Trigger PI3K/Akt and ERK1/2 signaling pathways; | NIH-3T3 cells; Rat model of full-thickness wound defect with bacterial infection | bacterial wound infections | [77] |
| BPNS | BP/4OI/Gel hydrogel | Antibacterial and antioxidant; Promote neovascularization | HUVECs; Rat model of diabetic wound infection | diabetic wound infections | [78] |
| BPNS | BP/Lid/Gel spray | NIR photothermal antibacterial; Promote the proliferation of endothelial cells; Promote neovascularization; Relieve pain by NIR-triggered Lid release | HUVECs; Rat model of diabetic wound infection | diabetic ulcer | [81] |
| BPNS | BP/AS/MSN-PEG spray | NIR photothermal antibacterial; Promote neovascularization; Reduce inflammation | HUVECs; Rat model of full-thickness wound defect with bacterial infection | bacterial wound infections | [82] |
3.2. BP-Based Scaffolds
3.2.1. BP Surface-Modified Scaffolds
3.2.2. BP Bulk-Doped Scaffolds

| Materials | Modification | Property | Therapy Mode | Application | Ref. |
|---|---|---|---|---|---|
| BPNS | BP/GO/PPF 3D printed scaffold | Improve cell adhesion and proliferation; Release phosphate continuously; Stimulate cell osteogenesis | MC3T3 cells; | cell proliferation and osteogenesis stimulation | [85] |
| BPNS | BP/ZnL2/HA/PLGA 3D printed scaffold | NIR photothermal antibacterial; Photothermal osteogenesis; Prevent the recurrence of the bone tumors | hBMSCs; Rat model of tibial defects | Infectious bone defects | [86] |
| BPNS | BP/BG 3D printed scaffold | Ablate osteosarcoma by photothermal effect; Improve bone regeneration | hBMSCs; Bone tumor-bearing nude mice | osteosarcoma | [87] |
| BPNS | BP/VEGF/DNA 3D printed scaffold | Enhance the mechanical strength; Accelerate vascular regeneration and bone regeneration. | BMSCs; HUVECs; Rat model of cranial defects | vascularized bone regeneration. | [88] |
| BPNS | BP/IBU/SrCL2/PLLAscaffold | Promote cell adhesion and proliferation; Photothermal-responsive release drug Promote biomineralization in vitro; Promote cell proliferation | MC3T3-E1 cells; | bone repair | [89] |
| BPNS | BP/PDA/Ag/CS/PCL scaffold | Promote the expression of osteogenesis-related proteins; Excellent photothermal antibacterial; | rBMSCs; Rat model of femoral defects | Infectious bone defects | [90] |
| BPNS | BP/DOX/PDA/Fs-NiTi scaffold | Sufficient mechanical strength; Controllable drug release behavior of NIR/pH-dual sensitivity; Photothermal chemotherapy and photothermal antibacterial; Promote bone regeneration | tumor cells (Saos-2 and MDA-MB-231); Rat model of ectopic osteosarcoma | Osteosarcoma; Infectious bone defects | [93] |
| BPNS | BP/PDA/Ti scaffold | Photothermal antibacterial; Promote bone regeneration | BMSCs; Rat model of tibial defects | Infectious bone defects | [94] |
| BPNS | BP/HA/Ti scaffold | Photothermal antibacterial; Promote bone regeneration | BMSCs; Rat model of tibial defects | Infectious bone defects | [95] |
| BPNS | BP/PDA/PLLA scaffold | Improve the stability of the BPNS; Improve cell adhesion and proliferation; promote osteogenic differentiation. | MG-63 cells | Infectious bone defects | [96] |
| BPNS | BP/BMP-2/PLGA scaffold | NIR photothermal antibacterial; Photothermal osteogenesis; | PDSCs; Rat model of cranial defects | Infectious bone defects | [97] |
| BPNS | BP/PCL scaffold | Excellent biocompatibility and high conductivity; Induction of angiogenesis and neurogenesis | Schwann cells; Rat model of neurological defect | peripheral nerve injury | [98] |
| BPNS | BP/HA/SiO2/PLLA 3D printed scaffold | Photothermal effect promotes the release of elements; Accelerate osteogenesis. | BMSCs; Rat model of cranial defects | bone repair; | [106] |
| BPNS | BP/HF 3D printed scaffold | Promote osteogenic stem cell proliferation, differentiation, and mineralization; Enhance vascularized bone regeneration; NIR-response repeatable shrinkage/swelling performance; | rBMSCs; Rat model of cranial defects | bone defects; tissue engineering repairs | [108] |
| BPNS | BP/β-TCP/DOX/BMP2 3D printed scaffold | Sufficient mechanical strength; Excellent photothermal effect; Control drug release; Reduce the long-term toxicity phenomenon of released DOX in vivo; Promote osteogenesis | rBMSCs; Bone tumor-bearing nude mice | tumor resection-induced tissue defects. | [109] |
| BPNS | BP/β-TCP/P/P(DLLA-TMC) 4D printed scaffold | Photothermal-responsive shape memory; Suitable mechanical properties; Promote bone regeneration by the continuous release of peptides | rBMSCs; Rat model of cranial defects | bone defects of irregular shapes. | [113] |
| BPNS | BP/PEEK/PTFE scaffold | Excellent antibacterial properties and wear resistance. | S. aureus | Infectious bone defects | [114] |
3.3. BP-Based Electrospun Fiber Membranes

| Materials | Modification | Property | Therapy Mode | Application | Ref. |
|---|---|---|---|---|---|
| BPQDs | BP/PCL/Col nanofiber | Promote cell attachment and proliferation; Improve osteogenic differentiation | MC3T3-E1 cells | osteodifferentiation enhancement | [120] |
| BPNS | BP/BMP2/PLLA nanofiber | Staged bone regeneration; Accelerate biomineralization; | BMSCs; Rat model of cranial defects | bone repair | [121] |
| BPNS | BP/VEGF/PLLA nanofiber | Promote osteogenic differentiation and angiogenesis | rBMSCs HUVECs | bone repair | [122] |
| BPNS | BP/PD nanofiber | Induce neurogenesis; Promote osteogenic differentiation; Stimulate Fanconi anemia pathway, | BMSCs; Schwann cells; Rat model of cranial defects | bone regeneration; neurogenesis | [126] |
| BPNS | BP/Apt19s-PCL nanofiber | Antibacterial through photothermal-triggered drug delivery; Accelerate biomineralization | MSCs Rat model of cranial defects | bone repair | [127] |
| BPNS | BP/Rg1/PLGA/Gel nanofiber | NIR photothermal antibacterial Facilitate the migration and tube formation of HUVECs Promote M2 polarization of macrophages; inhibit M1 polarization of macrophages. | 3T3 cells; HUVECs; Rat model of full-thickness wound defect with bacterial infection | wound healing | [128] |
| BPNS | BP/Hb/PLLA nanofiber | NIR-assisted oxygen delivery; Hemostasis; NIR photothermal antibacterial; Reduce inflammation; Promote cell proliferation, migration, and vascularization. | HUVECs Mouse fibroblasts (L929) Rat tail amputation Rat liver injury Rabbit liver injury models | wound healing | [129] |
| BPNS | BP/PLGA membrane. | Heat-induced osteogenesis; NIR photothermal response | Rat model of tibia defect | bone tissue engineering | [130] |
3.4. BP-Based Microspheres

3.5. BP-Based Microneedles
3.6. BP-Based Liposomes and Vesicles
| Materials | Modification | Property | Therapy Mode | Application | Ref. |
|---|---|---|---|---|---|
| BPNS | BP/SrCl2/PLGA microspheres | NIR-triggered drug delivery system; Improve bone regeneration by photothermal effect | Rat model of femoral defects | bone repair | [134] |
| BPQDs | BP/silk fibroin/gelatin microspheres | Promote neovascularization; NIR photothermal antibacterial; NIR-triggered drug delivery system | HUVECs; Rat model of full-thickness wound defect with bacterial infection | drug delivery and wound healing. | [135] |
| BPNS | BP/CS/bFGF/HA microspheres | Promote neovascularization and wound healing; NIR-triggered drug delivery system | HUVECs NIH/3T3 cells Rat model of diabetic wound infection | wound healing | [136] |
| BPQDs | BP/Hb separable microneedles | NIR responsive oxygen delivery; Promote wound healing | NIH 3T3 cells; Rat model of diabetic wound infection | drug delivery; wound healing | [143] |
| BPNS | BP/GT separable microneedles | NIR-regulated separable microneedles; Promote wound healing | NIH 3T3 cells; Rat model of systemic lupus erythematosus | systemic lupus erythematosus; drug delivery and wound healing | [144] |
| BP | BP/rapamycin microneedle balloon catheters | NIR-triggered drug delivery system Improved abdominal aortic restenosis | Rat model of abdominal aorta restenosis | wound healing | [145] |
| BP | BP/TP/Pae separable microneedles | NIR photothermal antibacterial; NIR-triggered drug delivery system; Relieve the fibrosis of the skin | NIH-3T3 cells; Rat model of early full-thickness skin wound | wound healing; vascular fibrosis | [146] |
| BPNS | BPNS/ROsi separable microneedles | NIR-triggered drug delivery system; Regulating the uniform distribution of the adipose tissue | mouse fibroblasts (L929) and human fibroblasts (HSF); C57 mice model of induced by a high-fat diet | wound healing; reduce weight | [147] |
| BPQDs | BP/vanco liposome | NIR photothermal antibacterial; NIR-triggered drug delivery system; | Staphylococcus aureus; Rat model of bacteria-infected subcutaneous abscess | antibiotic-resistant bacteria-caused skin abscess | [151] |
| BPQDs | BP/Ag+ vesicles | NIR-II photoacoustic imaging capability; NIR photothermal antibacterial; Photodynamic therapy | dendritic cells (DCs); bilateral4T1-tumor-bearing BALB/c mice. Rat model of bacteria-infected | immunization therapy; wound healing | [152] |
| BPQDs | BP/Apt/PLGA vesicles | Guide molecular recognition; NIR photothermal effect; Promote biomineralization | In vitro cell experiment; Rat model of cranial defects | bone repair | [153] |
| BPNS | BP/HNV vesicles | NIR photothermal antibacteria; NIR-triggered drug delivery system | RAW264.7 cells; L929 cells; Rat model of collagen-induced arthritis | bone arthritis | [154] |
4. Discussion
5. Prospects
- (a)
- BP composite biomedical materials exhibit excellent photothermal antibacterial properties and drug-release behavior, providing significant therapeutic advantages for challenging chronic wounds. With the development of precision medicine and smart healthcare concepts, intelligent BP-based diagnosis and treatment platforms that integrate advanced biosensing technologies for real-time monitoring, remote healthcare, and on-demand drug delivery represent an important future direction in this field.
- (b)
- BP readily oxidizes to form harmless phosphate compounds in the human body [155], possessing unique advantages in terms of biocompatibility. However, the long-term stability of BP-based composite biomedical materials is relatively poor during their preparation and storage processes. At present, the reported strategies to improve the stability of BP include physical encapsulation methods, such as coating with thin films of aluminum oxide, tin oxide, graphene, and hexagonal boron nitride [156], as well as surface chemical modification methods, such as polyethylene glycol [157], aromatic diazonium, metal ions, and metal–ligand modifications [158,159]. Nevertheless, most of these strategies face challenges related to high application costs, low protection efficiency, and complex operations. Developing economically efficient strategies to enhance the long-term stability of BP materials is a critical issue that urgently needs to be addressed for industrial applications. Innovative approaches, such as doping methods [160] or the application of biofilms [33], can be considered to make BP nanomaterials more practical for real-world use.
- (c)
- BP composite biomedical materials have demonstrated vast potential in the field of tissue engineering, addressing the non-self-supporting defects of BP nanomaterials. In dealing with irregular motion-type traumas, the integration of 3D and 4D printing technologies, electrospinning techniques, and layer-by-layer self-assembly techniques offers the prospect of producing BP composite biomedical materials that are better suited for clinical application. Adaptable control over processing techniques and materials based on the type of trauma allows for continued exploration of novel materials on the foundation of existing hydrogels, scaffolds, and fiber membranes. However, further exploration is needed on how to optimize the controllable preparation of BP nanomaterials and the production costs of BP composite medical formulations, as well as scaling up the production process. For example, although microsphere formulations can bypass the first-pass effect, improve bioavailability, and reduce drug dosage [161], they present high technological barriers, high development costs, and long cycles. Aside from addressing issues related to encapsulation rates and particle size uniformity, ensuring the sterility of the production process is also critical. Research into BP-based microsphere materials is limited, but future investigations in the fields of BP magnetic microspheres and bilayer microspheres can be pursued to enhance their performance [162]. Questions regarding whether BP-based microneedles will cause destructive damage to the skin and whether the voids formed in the skin are reversible remain to be verified. These factors have constrained the clinical application of microneedle technology, necessitating the establishment of unified standards for microneedle technology to enhance safety [163].
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4D | Four-dimensional |
| 3D | Three-dimensional |
| 2D | Two-dimensional |
| BP | Black phosphorus |
| BPNS | Black phosphorus nanosheets |
| BPQDs | Black Phosphorus quantum dots |
| NIR | Near infrared |
| PTT | Photothermal therapy |
| PDT | Photodynamics therapy |
| ROS | Reactive oxygen |
| OH | Oxhydryl |
| 1O2 | Singlet oxygen |
| ALP | Alkaline phosphatase |
| HSP | Heat shock protein |
| PO43− | Phosphate |
| OCN | Osteocalcin |
| BMP-2 | Bone morphogenetic protein-2 |
| Ag | Argentum |
| Zn | Zinc |
| SrCL2 | Strontium chloride |
| SiO2 | Silicon dioxide |
| Ni | Nickel |
| Ti | Titanium |
| Mg | Magnesium |
| VEGF | Vascular endothelial growth factor |
| bFGF | Basic fibroblast growth factor |
| β-TCP | β-Tricalcium phosphate |
| EGCG | Epigallocatechin gallate |
| 4OI | 4-Octylitaconic acid |
| AS | Astragalus IV |
| DFO | Deferoxamine |
| PRP | Platelet-rich plasma |
| Lid | Lidocaine |
| DOX | Doxorubicin |
| HA | Hydroxyapatite |
| Vanco | vancomycin |
| Apt | Aptamers |
| Rg1 | Ginsenoside Rg1 |
| Hb | Haemoglobin |
| E. coli | Escherichia coli |
| BS | Bacillus subtilis |
| S. aureus | Staphylococcus aureus |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| PEA | Polyesteramide |
| GelMA | Methacrylified gelatin |
| PVA | Polyvinyl alcohol |
| CS | Chitosan |
| Gel | Gelatin |
| Col | Collagen |
| HA | Hyaluronic acid |
| GelMA | Methacrylated gelatin |
| MSN | Mesoporous silica nanoparticles |
| PEG | Polyethylene glycol |
| CNT | Carbon nanotubes |
| OPF | Fumaric acid |
| Alg | Sodium alginate |
| PPy | Polypyrrole |
| PCL | Polycaprolactone |
| PLGA | Polylactic acid–glycolic acid copolymer |
| PLA | Polylactic acid |
| GO | Graphene |
| PPF | Polypropylene glycol fumarate |
| BG | Bioglass |
| PLLA | Polylactic acid |
| DLLA-TMC | Poly(lactic acid–co-trimethylene carbonate) |
| PPS | Polypropylene sulfide |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PSS | Polystyrene sulfonate |
| PAA | Polyacrylic acid |
| BMSCs | Bone marrow mesenchymal stem cells |
| HDPSCs | Human pulp stem cells |
| Saos-2 | Osteosarcoma cells |
| HBMSCs | Human bone marrow mesenchymal stem cells |
| NIH/3T3 | Mouse embryonic cells |
| MC3T3 | Mouse embryonic osteoblasts |
| MC3T3-E1 | Mouse embryonic osteoblast precursor cells |
| HUVECs | Human umbilical vein endothelial cells |
| NSCs | Neural stem cells |
| MSCs | Mesenchymal stem cells |
| DC | Dendritic cells |
| MDA-MB-231 | Human breast cancer cells |
| MG -63 | Human osteosarcoma cells |
| hBM-MSCs | human bone marrow mesenchymal stem cells/stromal cells |
| Nrf2 | Nuclear factor E2-like 2 |
| BMP-RUNX2 | Bone Morphogenic Protein–runt-related transcription factor 2 |
| PI3K-AKT | Phosphatidylinositide 3 kinases-serine/threonine kinase |
| HSC | Hematopoietic stem cells |
References
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hui, K.S.; Hui, K.N. 2D Black Phosphorus: From Preparation to Applications for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700491. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, Z.; Shi, X.; Zhang, K.; Zhang, H. Recent progress in black phosphorus and black-phosphorus-analogue materials: Properties, synthesis and applications. Nanoscale 2019, 11, 14491–14527. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Jin, H.; Wang, Z.; Li, J. Black phosphorus quantum dots: Synthesis, properties, functionalized modification and applications. Chem. Soc. Rev. 2018, 47, 6795–6823. [Google Scholar] [CrossRef]
- Lei, W.; Liu, G.; Zhang, J.; Liu, M. Black phosphorus nanostructures: Recent advances in hybridization, doping and functionalization. Chem. Soc. Rev. 2017, 46, 3492–3509. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black Phosphorus and its Biomedical Applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Q.; Tong, Y.; Wang, J. Light-Induced Ambient Degradation of Few-Layer Black Phosphorus: Mechanism and Protection. Angew. Chem. Int. Ed. 2016, 55, 11437–11441. [Google Scholar] [CrossRef]
- Thurakkal, S.; Zhang, X. Recent Advances in Chemical Functionalization of 2D Black Phosphorous Nanosheets. Adv. Sci. 2020, 7, 1902359. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cen, Y.; Huang, J.; Li, X.; Zhang, H.; Geng, Y.; Yakobson, B.I.; Du, Y.; Tian, X. Zinc oxide-black phosphorus composites for ultrasensitive nitrogen dioxide sensing. Nanoscale Horiz. 2018, 3, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Jeong, R.H.; Lee, J.W.; Kim, D.I.; Yang, J.W.; Park, S.; Boo, J.-H. Black phosphorus @ molybdenum disulfide 2D nanocomposite with broad light absorption and high stability for methylene blue decomposition photocatalyst. Nanotechnology 2020, 31, 155704. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, S.; Chaves, A.; Song, C.; Özçelik, V.O.; Low, T.; Yan, H. Infrared fingerprints of few-layer black phosphorus. Nat. Commun. 2017, 8, 14071. [Google Scholar] [CrossRef]
- Ahmed, T.; Tahir, M.; Low, M.X.; Ren, Y.; Tawfik, S.A.; Mayes, E.L.H.; Kuriakose, S.; Nawaz, S.; Spencer, M.J.S.; Chen, H.; et al. Fully Light-Controlled Memory and Neuromorphic Computation in Layered Black Phosphorus. Adv. Mater. 2021, 33, 2004207. [Google Scholar] [CrossRef]
- Hu, Y.; Ji, Q.; Huang, M.; Chang, L.; Zhang, C.; Wu, G.; Zi, B.; Bao, N.; Chen, W.; Wu, Y. Light-Driven Self-Oscillating Actuators with Phototactic Locomotion Based on Black Phosphorus Heterostructure. Angew. Chem. Int. Ed. 2021, 60, 20511–20517. [Google Scholar] [CrossRef]
- Shao, J.; Ruan, C.; Xie, H.; Chu, P.K.; Yu, X. Photochemical Activity of Black Phosphorus for Near-Infrared Light Controlled In Situ Biomineralization. Adv. Sci. 2020, 7, 2000439. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, Y.; Zhou, B.; Zhang, Y.; Wu, J.; Hu, R.; Liu, L.; Song, J.; Qu, J. Enhanced photocatalytic performance of Ag/TiO2 nanohybrid sensitized by black phosphorus nanosheets in visible and near-infrared light. J. Colloid Interface Sci. 2019, 534, 1–11. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy. Available online: https://pubmed.ncbi.nlm.nih.gov/34575419/ (accessed on 12 February 2023).
- Shao, J.; Ruan, C.; Xie, H.; Li, Z.; Wang, H.; Chu, P.K.; Yu, X.-F. Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv. Sci. 2018, 5, 1700848. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black Phosphorus Nanosheets Immobilizing Ce6 for Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 12431–12440. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ouyang, J.; Yi, X.; Xu, Y.; Niu, C.; Zhang, W.; Wang, L.; Sheng, J.; Deng, L.; Liu, Y.; et al. Black Phosphorus Nanosheets as a Neuroprotective Nanomedicine for Neurodegenerative Disorder Therapy. Adv. Mater. 2018, 30, 1703458. [Google Scholar] [CrossRef]
- Jin, L.; Hu, P.; Wang, Y.; Wu, L.; Qin, K.; Cheng, H.; Wang, S.; Pan, B.; Xin, H.; Zhang, W.; et al. Fast-Acting Black-Phosphorus-Assisted Depression Therapy with Low Toxicity. Adv. Mater. 2020, 32, 1906050. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Li, Z.; Liu, Y.; Wang, Q.; Luo, J.; Chen, X.; Xie, Z.; Zhang, Y.; Zhang, H.; Chen, T. Brain-targeted delivery shuttled by black phosphorus nanostructure to treat Parkinson’s disease. Biomaterials 2020, 260, 120339. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Li, Z.; Kang, Y.; Su, Z.; Yu, R.; Zhang, S. Black phosphorus quantum dots encapsulated in anionic waterborne polyurethane nanoparticles for enhancing stability and reactive oxygen species generation for cancer PDT/PTT therapy. J. Mater. Chem. B 2020, 8, 10650–10661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Cui, Y.; Zhou, N.; Shen, J. Near-infrared light-mediated photodynamic/photothermal therapy nanoplatform by the assembly of Fe3O4 carbon dots with graphitic black phosphorus quantum dots. Int. J. Nanomed. 2018, 13, 2803–2819. [Google Scholar] [CrossRef]
- Guo, T.; Wu, Y.; Lin, Y.; Xu, X.; Lian, H.; Huang, G.; Liu, J.-Z.; Wu, X.; Yang, H.-H. Black Phosphorus Quantum Dots with Renal Clearance Property for Efficient Photodynamic Therapy. Small 2018, 14, 1702815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lin, X.; Lan, S.; Sun, H.; Wang, X.; Liu, X.; Zhang, Y.; Zeng, Y. Localized Surface Plasmon Resonance Enhanced Singlet Oxygen Generation and Light Absorption Based on Black Phosphorus@AuNPs Nanosheet for Tumor Photodynamic/Thermal Therapy. Part. Part. Syst. Charact. 2018, 35, 1800010. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, X.; Zhang, S.; Lei, L.; Ma, W.; Li, D.; Wang, W.; Zhao, Q.; Xing, B. Bacterial toxicity of exfoliated black phosphorus nanosheets. Ecotoxicol. Environ. Saf. 2018, 161, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lai, C.; Zeng, G.; Huang, D.; Qin, L.; Zhang, M.; Cheng, M.; Liu, X.; Yi, H.; Zhou, C.; et al. Black Phosphorus, a Rising Star 2D Nanomaterial in the Post-Graphene Era: Synthesis, Properties, Modifications, and Photocatalysis Applications. Small 2019, 15, 1804565. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef]
- Tayari, V.; Hemsworth, N.; Fakih, I.; Favron, A.; Gaufrès, E.; Gervais, G.; Martel, R.; Szkopek, T. Two-dimensional magnetotransport in a black phosphorus naked quantum well. Nat. Commun. 2015, 6, 7702. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, M.; Chu, X.; Zhang, Q.; Su, Y.; Sun, B.; Lu, T.; Zhou, N.; Zhang, J.; Wang, J.; et al. Black phosphorus nanosheets-based nanocarriers for enhancing chemotherapy drug sensitiveness via depleting mutant p53 and resistant cancer multimodal therapy. Chem. Eng. J. 2019, 370, 387–399. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef]
- Liang, X.; Ye, X.; Wang, C.; Xing, C.; Miao, Q.; Xie, Z.; Chen, X.; Zhang, X.; Zhang, H.; Mei, L. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control Release 2019, 296, 150–161. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, L.; Liu, Y.; Xu, X.; Xing, C.; Wang, M.; Bai, S.-M.; Lu, C.-H.; Yang, H.-H. A black phosphorus nanosheet-based siRNA delivery system for synergistic photothermal and gene therapy. Chem. Commun. 2018, 54, 3142–3145. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, H.; Shao, J.; Jiang, C.; Zhang, F.; Lin, J.; Zhang, H.; Li, J.; Huang, P. Polydopamine-functionalized black phosphorus quantum dots for cancer theranostics. Appl. Mater. Today 2019, 15, 297–304. [Google Scholar] [CrossRef]
- Wang, X.; Jones, A.M.; Seyler, K.L.; Tran, V.; Jia, Y.; Zhao, H.; Wang, H.; Yang, L.; Xu, X.; Xia, F. Highly anisotropic and robust excitons in monolayer black phosphorus. Nat. Nanotechnol. 2015, 10, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, Y.; Chen, L.; Zhu, T.; Ye, K.; Jia, C.; Wang, H.; Zhu, M.; Fan, C.; Mo, X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019, 84, 98–113. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Chen, N.; Ramakrishna, S.; Mo, X. The cellular response of nerve cells on poly-l-lysine coated PLGA-MWCNTs aligned nanofibers under electrical stimulation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 715–726. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Y.; Xiao, C.; Zhao, W.; Wu, H.; Tang, J.; Li, Z.; Yu, S.; Li, X.; Min, L.; et al. Application of hydroxyapatite nanoparticles in tumor-associated bone segmental defect. Sci. Adv. 2019, 5, eaax6946. [Google Scholar] [CrossRef]
- Comber, S.; Gardner, M.; Georges, K.; Blackwood, D.; Gilmour, D. Domestic source of phosphorus to sewage treatment works. Environ. Technol. 2013, 34, 1349–1358. [Google Scholar] [CrossRef]
- Lee, H.U.; Park, S.Y.; Lee, S.C.; Choi, S.; Seo, S.; Kim, H.; Won, J.; Choi, K.; Kang, K.S.; Park, H.G.; et al. Black Phosphorus (BP) Nanodots for Potential Biomedical Applications. Small 2016, 12, 214–219. [Google Scholar] [CrossRef]
- Shin, Y.C.; Song, S.-J.; Lee, Y.B.; Kang, M.S.; Lee, H.U.; Oh, J.-W.; Han, D.-W. Application of black phosphorus nanodots to live cell imaging. Biomater. Res. 2018, 22, 31. [Google Scholar] [CrossRef]
- Liu, X.; Bai, Y.; Xu, J.; Xu, Q.; Xiao, L.; Sun, L.; Weng, J.; Zhao, Y. Robust Amphiphobic Few-Layer Black Phosphorus Nanosheet with Improved Stability. Adv. Sci. 2019, 6, 1901991. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wu, J.; Gu, Z. Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Appl. Mater. Interfaces 2019, 11, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Doganov, R.A.; O’Farrell, E.C.T.; Koenig, S.P.; Yeo, Y.; Ziletti, A.; Carvalho, A.; Campbell, D.K.; Coker, D.F.; Watanabe, K.; Taniguchi, T.; et al. Transport properties of pristine few-layer black phosphorus by van der Waals passivation in an inert atmosphere. Nat. Commun. 2015, 6, 6647. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, T.; Feng, X.; Zhou, Y.; Chen, M.; Wang, W.; Zhao, Y.; Lu, C.; Quan, G.; Cai, J.; et al. A Perfect Pair: Stabilized Black Phosphorous Nanosheets Engineering with Antimicrobial Peptides for Robust Multidrug Resistant Bacteria Eradication. Adv. Healthc. Mater. 2022, 11, e2101846. [Google Scholar] [CrossRef]
- Guo, T.; Zhuang, S.; Qiu, H.; Yating, G. Black Phosphorus Nanosheets for Killing Bacteria through Nanoknife Effect. Part. Part. Syst. Charact. 2020, 37, 2000169. [Google Scholar] [CrossRef]
- Shaw, Z.L.; Kuriakose, S.; Cheeseman, S.; Mayes, E.L.H.; Murali, A.; Oo, Z.Y.; Ahmed, T.; Tran, N.; Boyce, K.; Chapman, J.; et al. Broad-Spectrum Solvent-free Layered Black Phosphorus as a Rapid Action Antimicrobial. ACS Appl. Mater. Interfaces 2021, 13, 17340–17352. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Zheng, Y.; Wang, X.; Wu, S. In Situ Disinfection through Photoinspired Radical Oxygen Species Storage and Thermal-Triggered Release from Black Phosphorous with Strengthened Chemical Stability. Small 2018, 14, 1703197. [Google Scholar] [CrossRef]
- Wang, S.-G.; Chen, Y.-C.; Chen, Y.-C. Antibacterial gold nanoparticle-based photothermal killing of vancomycin-resistant bacteria. Nanomedicine 2018, 13, 1405–1416. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, X.-F.; Zhu, C.-L.; Yang, H.-J.; Lu, C.-H.; Wang, W.-L.; Pang, Y.; Yang, C.; Chen, L.-J.; Li, X.-F. A Stable Quaternized Chitosan-Black Phosphorus Nanocomposite for Synergetic Disinfection of Antibiotic-Resistant Pathogens. ACS Appl. Bio Mater. 2021, 4, 4821–4832. [Google Scholar] [CrossRef]
- He, F.; Cheng, K.; Qi, J.; He, F.; Chu, C.; Xiong, Y.; Zhao, J.; Ding, J.; Kong, F.; Cao, Z.; et al. Black phosphorus nanosheets enhance differentiation of neural progenitor cells for improved treatment in spinal cord injury. Chem. Eng. J. 2023, 472, 144977. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, J.; Wu, B.; Shang, Y.; Huang, X.; Dong, H.; Liu, H.; Gui, R.; Nie, X. A Nano-Traditional Chinese Medicine Against Lymphoma That Regulates the Level of Reactive Oxygen Species. Front. Chem. 2020, 8, 565. [Google Scholar] [CrossRef]
- He, J.; Chen, G.; Zhao, P.; Ou, C. Near-infrared light-controllable bufalin delivery from a black phosphorus-hybrid supramolecular hydrogel for synergistic photothermal-chemo tumor therapy. Nano Res. 2021, 14, 3988–3998. [Google Scholar] [CrossRef]
- Huang, Y.; Li, R.; Dai, Y.; Qin, C.; He, J.; Yang, S.; Wang, T.; Su, Y.; Jia, L.; Zhao, W. Rhamnolipid-assisted black phosphorus nanosheets with efficient isolinderalactone loading against drug resistant Helicobacter pylori. Mater. Des. 2022, 216, 110536. [Google Scholar] [CrossRef]
- Naito, H.; Yoshimura, M.; Mizuno, T.; Takasawa, S.; Tojo, T.; Taniguchi, S. The advantages of three-dimensional culture in a collagen hydrogel for stem cell differentiation: Advantages of 3D Culture in a Collagen Hydrogel. J. Biomed. Mater. Res. A 2013, 101, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, C.; Xie, J.; Ågren, H.; Zhang, H. Black Phosphorus/Polymers: Status and Challenges. Adv. Mater. 2021, 33, 2100113. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- de Faro Silva, R.; Barreto, A.S.; Trindade, G.d.G.G.; Lima, C.M.; Araújo, A.A.d.S.; Menezes, I.R.A.; Candido, E.A.F.; Santana, É.T.N.; Silva-Júnior, W.M.; Quintans, J.S.S.; et al. Enhancement of the functionality of women with knee osteoarthritis by a gel formulation with Caryocar coriaceum Wittm (“Pequi”) nanoencapsulated pulp fixed oil. Biomed. Pharmacother. 2022, 150, 112938. [Google Scholar] [CrossRef] [PubMed]
- Trbakovic, A.; Hedenqvist, P.; Mellgren, T.; Ley, C.; Hilborn, J.; Ossipov, D.; Ekman, S.; Johansson, C.B.; Jensen-Waern, M.; Thor, A. A new synthetic granular calcium phosphate compound induces new bone in a sinus lift rabbit model. J. Dent. 2018, 70, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Zeng, M.; Xia, Q.; Wu, S.; Ye, S.; Rao, J.; Lin, D.; Zhang, H.; Ma, H.; Han, Z.; et al. Efficacy and safety of umbilical cord mesenchymal stem cells in treatment of cesarean section skin scars: A randomized clinical trial. Stem Cell Res. Ther. 2020, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Meuli, M.; Hartmann-Fritsch, F.; Hüging, M.; Marino, D.; Saglini, M.; Hynes, S.; Neuhaus, K.; Manuel, E.; Middelkoop, E.; Reichmann, E.; et al. A Cultured Autologous Dermo-epidermal Skin Substitute for Full-Thickness Skin Defects: A Phase I, Open, Prospective Clinical Trial in Children. Plast. Reconstr. Surg. 2019, 144, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.A. Study on Black Phosphorus Nanosheets/Nano Magnesium Oxide Incorporated Composite Hydrogels for Promoting Bone Repair and Related Mechanisms. Ph.D. Thesis, Bethune Second Clinical School of Jilin University, Changchun, China, 2022. [Google Scholar]
- Miao, Y.; Shi, X.; Li, Q.; Hao, L.; Liu, L.; Liu, X.; Chen, Y.; Wang, Y. Engineering natural matrices with black phosphorus nanosheets to generate multi-functional therapeutic nanocomposite hydrogels. Biomater. Sci. 2019, 7, 4046–4059. [Google Scholar] [CrossRef]
- Xu, D.; Gan, K.; Wang, Y.; Wu, Z.; Wang, Y.; Zhang, S.; Peng, Y.; Fang, X.; Wei, H.; Zhang, Y.; et al. A Composite Deferoxamine/Black Phosphorus Nanosheet/Gelatin Hydrogel Scaffold for Ischemic Tibial Bone Repair. Int. J. Nanomed. 2022, 17, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, J.; Tang, W.; Hu, L.; Chen, X.; Su, Y.; Zou, C.; Wang, J.; Lu, W.W.; Zhen, W.; et al. Multifunctional Nanoengineered Hydrogels Consisting of Black Phosphorus Nanosheets Upregulate Bone Formation. Small 2019, 15, 1901560. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, C.; He, L.; Zhou, J.; Chen, T.; Ouyang, L.; Guo, X.; Qu, Y.; Luo, Z.; Duan, D. Stratified-structural hydrogel incorporated with magnesium-ion-modified black phosphorus nanosheets for promoting neuro-vascularized bone regeneration. Bioact. Mater. 2022, 16, 271–284. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Liu, X.; George, M.N.; Li, L.; Gamble, D.; Miller, A.L., II; Gaihre, B.; Waletzki, B.E.; Lu, L. Injectable Electrical Conductive and Phosphate Releasing Gel with Two-Dimensional Black Phosphorus and Carbon Nanotubes for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 4653–4665. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Vialli, N.; Fuh, J.Y.H.; Lu, W.F. Conductive collagen/polypyrrole-b-polycaprolactone hydrogel for bioprinting of neural tissue constructs. Int. J. Bioprinting 1970, 5, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Manzari-Tavakoli, A.; Tarasi, R.; Sedghi, R.; Moghimi, A.; Niknejad, H. Fabrication of nanochitosan incorporated polypyrrole/alginate conducting scaffold for neural tissue engineering. Sci. Rep. 2020, 10, 22012. [Google Scholar] [CrossRef]
- Pan, W.; Dai, C.; Li, Y.; Yin, Y.; Gong, L.; Machuki, J.O.; Yang, Y.; Qiu, S.; Guo, K.; Gao, F. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials 2020, 239, 119851. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, L.; Wei, C.; Guo, R. A bioactive dextran-based hydrogel promote the healing of infected wounds via antibacterial and immunomodulatory. Carbohydr. Polym. 2022, 291, 119558. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. Zinc-Modified Sulfonated Polyetheretherketone Surface with Immunomodulatory Function for Guiding Cell Fate and Bone Regeneration. Adv. Sci. 2018, 5, 1800749. [Google Scholar] [CrossRef]
- Huang, S.; Xu, S.; Hu, Y.; Zhao, X.; Chang, L.; Chen, Z.; Mei, X. Preparation of NIR-responsive, ROS-generating and antibacterial black phosphorus quantum dots for promoting the MRSA-infected wound healing in diabetic rats. Acta Biomater. 2022, 137, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chang, L.; Hu, Y.; Zhao, X.; Huang, S.; Chen, Z.; Ren, X.; Mei, X. Tea polyphenol modified, photothermal responsive and ROS generative black phosphorus quantum dots as nanoplatforms for promoting MRSA infected wounds healing in diabetic rats. J. Nanobiotechnology 2021, 19, 362. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Li, Z.; Zhu, S.; Zheng, Y.; Yeung, K.W.K.; Wu, S. Repeatable Photodynamic Therapy with Triggered Signaling Pathways of Fibroblast Cell Proliferation and Differentiation To Promote Bacteria-Accompanied Wound Healing. ACS Nano 2018, 12, 1747–1759. [Google Scholar] [CrossRef]
- Ding, Q.; Sun, T.; Su, W.; Jing, X.; Ye, B.; Su, Y.; Zeng, L.; Qu, Y.; Yang, X.; Wu, Y.; et al. Bioinspired Multifunctional Black Phosphorus Hydrogel with Antibacterial and Antioxidant Properties: A Stepwise Countermeasure for Diabetic Skin Wound Healing. Adv. Healthc. Mater. 2022, 11, 2102791. [Google Scholar] [CrossRef]
- Prabhu, D.; Ravikumar, P. Novel user-friendly night care spray to manage skin darkening. J. Cosmet. Dermatol. 2020, 19, 1439–1446. [Google Scholar] [CrossRef]
- Plefh, A.C.V.; Hoshino, L.V.C.; Sato, F.; Castilha, L.D.; Santos, T.C.; Vital, A.C.P.; Matumoto-Pintro, P.T. Cloves (Syzygium aromaticum) fluid gel on healing of pododermatitis in rabbits. Vet. Res. Commun. 2021, 45, 293–304. [Google Scholar] [CrossRef]
- Ouyang, J.; Ji, X.; Zhang, X.; Feng, C.; Tang, Z.; Kong, N.; Xie, A.; Wang, J.; Sui, X.; Deng, L.; et al. In situ sprayed NIR-responsive, analgesic black phosphorus-based gel for diabetic ulcer treatment. Proc. Natl. Acad. Sci. USA 2020, 117, 28667–28677. [Google Scholar] [CrossRef]
- Liu, L.; Wang, W.; Hong, W.; Jin, Y.; Wang, L.; Liu, S.; Wang, A.; Liu, X. Photothermal 2D Nanosheets Combined with Astragaloside IV for Antibacterial Properties and Promoting Angiogenesis to Treat Infected Wounds. Front. Bioeng. Biotechnol. 2022, 9, 826011. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.; Diez-Escudero, A.; Maazouz, Y.; Rappe, K.; Espanol, M.; Montufar, E.B.; Bonany, M.; Sadowska, J.M.; Guillem-Marti, J.; Öhman-Mägi, C.; et al. Osteoinduction by Foamed and 3D-Printed Calcium Phosphate Scaffolds: Effect of Nanostructure and Pore Architecture. ACS Appl. Mater. Interfaces 2017, 9, 41722–41736. [Google Scholar] [CrossRef]
- Guerra, A.J.; Lammel-Lindemann, J.; Katko, A.; Kleinfehn, A.; Rodriguez, C.A.; Catalani, L.H.; Becker, M.L.; Ciurana, J.; Dean, D. Optimization of photocrosslinkable resin components and 3D printing process parameters. Acta Biomater. 2019, 97, 154–161. [Google Scholar] [CrossRef]
- Liu, X.; Miller, A.L.; Park, S.; George, M.N.; Waletzki, B.E.; Xu, H.; Terzic, A.; Lu, L. Two-Dimensional Black Phosphorus and Graphene Oxide Nanosheets Synergistically Enhance Cell Proliferation and Osteogenesis on 3D Printed Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 23558–23572. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liao, Q.; Wu, L.; Luo, Y.; Zhang, W.; Guan, M.; Pan, H.; Tong, L.; Chu, P.K.; Wang, H. ZnL2-BPs Integrated Bone Scaffold under Sequential Photothermal Mediation: A Win-Win Strategy Delivering Antibacterial Therapy and Fostering Osteogenesis Thereafter. ACS Nano 2021, 15, 17854–17869. [Google Scholar] [CrossRef]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds: A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Y.; Luo, J.; Liu, X.; Yang, Q.; Shi, X.; Wang, Y. Black phosphorus nanosheets-enabled DNA hydrogel integrating 3D-printed scaffold for promoting vascularized bone regeneration. Bioact. Mater. 2023, 21, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, R.; Liang, Q.; Xiao, X. Multifunctional modified polylactic acid nanofibrous scaffold incorporating sodium alginate microspheres decorated with strontium and black phosphorus for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2021, 32, 1598–1617. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, X.; Wang, D.; Zhang, H.; Xin, Q.; Wu, M.; Xu, X.; Sun, F.; Xing, Z.; Wang, L.; et al. Chloroplast-inspired Scaffold for Infected Bone Defect Therapy: Towards Stable Photothermal Properties and Self-Defensive Functionality. Adv. Sci. 2022, 9, e2204535. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, S.; Tong, S.; Guo, S. Study on Exosomes Promoting the Osteogenic Differentiation of ADSCs in Graphene Porous Titanium Alloy Scaffolds. Front. Bioeng. Biotechnol. 2022, 10, 905511. [Google Scholar] [CrossRef]
- ScienceDirect. Current Advances Concerning the Most Cited Metal Ions Doped Bioceramics and Silicate-Based Bioactive Glasses for Bone Tissue Engineering. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/abs/pii/S0272884220329175?via%3Dihub (accessed on 6 August 2023).
- Ma, Y.; Jiang, L.; Hu, J.; Zhu, E.; Zhang, N. Developing a Versatile Multiscale Therapeutic Platform for Osteosarcoma Synergistic Photothermo-Chemotherapy with Effective Osteogenicity and Antibacterial Capability. ACS Appl. Mater. Interfaces 2022, 14, 44065–44083. [Google Scholar] [CrossRef]
- Zeng, J.; Gu, C.; Geng, X.; Lin, K.; Xie, Y.; Chen, X. Combined photothermal and sonodynamic therapy using a 2D black phosphorus nanosheets loaded coating for efficient bacterial inhibition and bone-implant integration. Biomaterials 2023, 297, 122122. [Google Scholar] [CrossRef]
- Yuan, B.; Zhou, X.; Li, Y.; Zhao, Y.; Xue, M.; Guo, Q.; Zheng, G.; Chen, X.; Lin, H.; Guo, X. Black-Phosphorus-Nanosheet-Reinforced Coating of Implants for Sequential Biofilm Ablation and Bone Fracture Healing Acceleration. ACS Appl. Mater. Interfaces 2022, 14, 47036–47051. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qian, G.; Yao, J.; Cai, W.; Peng, S.; Shuai, C. Polydopamine-decorated black phosphorous to enhance stability in polymer scaffold. Nanotechnology 2021, 32, 455701. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Zhang, J.; Zhong, H.; Liang, J.; Huang, S.; Liao, G.; Zhang, B.; Liu, C. Fabrication and evaluation of bone morphogenetic protein-2 microspheres coated black phosphorus nanosheets@polylactic-glycolic acid copolymers scaffold: A multifunctional antibacterial photothermal scaffold for bone regeneration. Int. J. Biol. Macromol. 2022, 210, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yuan, W.-E.; Cheng, Y.; Yang, Y.; Qu, X.; Fan, C. Concentrically Integrative Bioassembly of a Three-Dimensional Black Phosphorus Nanoscaffold for Restoring Neurogenesis, Angiogenesis, and Immune Homeostasis. Nano Lett. 2019, 19, 8990–9001. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.; Garrudo, F.F.F.; Marques, A.C.; Cabral, J.M.S.; Morgado, J.; Ferreira, F.C.; Silva, J.C. Novel Electroactive Mineralized Polyacrylonitrile/PEDOT:PSS Electrospun Nanofibers for Bone Repair Applications. Int. J. Mol. Sci. 2023, 24, 13203. [Google Scholar] [CrossRef] [PubMed]
- Sordini, L.; Silva, J.C.; Garrudo, F.F.F.; Rodrigues, C.A.V.; Marques, A.C.; Linhardt, R.J.; Cabral, J.M.S.; Morgado, J.; Ferreira, F.C. PEDOT:PSS-Coated Polybenzimidazole Electroconductive Nanofibers for Biomedical Applications. Polymers 2021, 13, 2786. [Google Scholar] [CrossRef]
- Han, S.; Zhang, Q.; Zhang, X.; Liu, X.; Lu, L.; Wei, J.; Li, Y.; Wang, Y.; Zheng, G. A digital microfluidic diluter-based microalgal motion biosensor for marine pollution monitoring. Biosens. Bioelectron. 2019, 143, 111597. [Google Scholar] [CrossRef]
- Tang, Z.; Kong, N.; Zhang, X.; Liu, Y.; Hu, P.; Mou, S.; Liljeström, P.; Shi, J.; Tan, W.; Kim, J.S.; et al. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 2020, 5, 847–860. [Google Scholar] [CrossRef]
- Chowdhury, M.S.; Zheng, W.; Kumari, S.; Heyman, J.; Zhang, X.; Dey, P.; Weitz, D.A.; Haag, R. Dendronized fluorosurfactant for highly stable water-in-fluorinated oil emulsions with minimal inter-droplet transfer of small molecules. Nat. Commun. 2019, 10, 4546. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Khalid, N.; Neves, M.A.; Kuroiwa, T.; Nakajima, M.; Uemura, K.; Ichikawa, S.; Kobayashi, I. Industrial lab-on-a-chip: Design, applications and scale-up for drug discovery and delivery. Adv. Drug Deliv. Rev. 2013, 65, 1626–1663. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Zheng, G.; Zhang, X. Microenvironment-Controlled Micropatterned Microfluidic Model (MMMM) for Biomimetic In Situ Studies. ACS Nano 2020, 14, 9861–9872. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Ouyang, J.; Chu, D.; Han, F.; Shi, L.; Liu, R.; Guo, Z.; Gu, G.X.; Tao, W.; et al. Ca2+-supplying black phosphorus-based scaffolds fabricated with microfluidic technology for osteogenesis. Bioact. Mater. 2021, 6, 4053–4064. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.; Yang, C.; Shao, C.; Shi, K.; Shang, L.; Ye, F.; Zhao, Y. Microfluidic 3D Printing Responsive Scaffolds with Biomimetic Enrichment Channels for Bone Regeneration. Adv. Funct. Mater. 2021, 31, 2105190. [Google Scholar] [CrossRef]
- Wang, C.; Ye, X.; Zhao, Y.; Bai, L.; He, Z.; Tong, Q.; Xie, X.; Zhu, H.; Cai, D.; Zhou, Y.; et al. Cryogenic 3D printing of porous scaffolds for in situ delivery of 2D black phosphorus nanosheets, doxorubicin hydrochloride and osteogenic peptide for treating tumor resection-induced bone defects. Biofabrication 2020, 12, 035004. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001. [Google Scholar] [CrossRef] [PubMed]
- Lui, Y.S.; Sow, W.T.; Tan, L.P.; Wu, Y.; Lai, Y.; Li, H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater. 2019, 92, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Liu, J.; Zhao, Q.; He, Z.; Li, K.; Lu, B.; Huang, W.; Wei, Y.; Tang, Y.; et al. Advanced reconfigurable scaffolds fabricated by 4D printing for treating critical-size bone defects of irregular shapes. Biofabrication 2020, 12, 045025. [Google Scholar] [CrossRef]
- Sun, X.; Yu, C.; Zhang, L.; Cao, J.; Kaleli, E.H.; Xie, G. Tribological and Antibacterial Properties of Polyetheretherketone Composites with Black Phosphorus Nanosheets. Polymers 2022, 14, 1242. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Lo, T.-S.; Lin, Y.-T.; Chien, Y.-H.; Lu, C.-J.; Liu, S.-J. Fabrication of Drug-Eluting Polycaprolactone/poly(lactic-co-glycolic Acid) Prolapse Mats Using Solution-Extrusion 3D Printing and Coaxial Electrospinning Techniques. Polymers 2021, 13, 2295. [Google Scholar] [CrossRef]
- Bao, X.; Zhu, Q.; Chen, Y.; Tang, H.; Deng, W.; Guo, H.; Zeng, L. Antibacterial and antioxidant films based on HA/Gr/TA fabricated using electrospinning for wound healing. Int. J. Pharm. 2022, 626, 122139. [Google Scholar] [CrossRef]
- Liu, Q.; Jia, H.; Ouyang, W.; Mu, Y.; Wu, Z. Fabrication of Antimicrobial Multilayered Nanofibrous Scaffolds-Loaded Drug via Electrospinning for Biomedical Application. Front. Bioeng. Biotechnol. 2021, 9, 755777. [Google Scholar] [CrossRef]
- Chen, J.; Shu, L.P.; Li, X.Z.; Liu, Q.; Wu, Y.; Liu, Y.; Wang, W.Y.; Liu, Y.; Ye, C.; Ma, M.X. PLA-hydroxyl acetate copolymer loaded human BMSCs to construct tissue engineered bone. Chin. J. Tissue Eng. Res. 2019, 23, 4109–4114. [Google Scholar]
- Zhu, Y.; Li, D.; Zhang, K.; Jiang, L.; Shi, C.; Fangteng, J.; Zheng, C.; Yang, B.; Sun, H. Novel Synthesized Nanofibrous Scaffold Efficiently Delivered hBMP-2 Encoded in Adenoviral Vector to Promote Bone Regeneration. J. Biomed. Nanotechnol. 2017, 13, 437–446. [Google Scholar] [CrossRef]
- Lee, Y.B.; Song, S.-J.; Shin, Y.C.; Jung, Y.J.; Kim, B.; Kang, M.S.; Kwon, I.K.; Hyon, S.-H.; Lee, H.U.; Jung, S.-H.; et al. Ternary nanofiber matrices composed of PCL/black phosphorus/collagen to enhance osteodifferentiation. J. Ind. Eng. Chem. 2019, 80, 802–810. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Z.; Cai, Z.; Zhao, J.; Lu, M.; Liang, J.; Wang, F.; Qi, J.; Cui, W.; Deng, L. Bioinspired Functional Black Phosphorus Electrospun Fibers Achieving Recruitment and Biomineralization for Staged Bone Regeneration. Small 2020, 16, 2005433. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, J.; Wu, L.; Xi, K.; Xin, T.; Tang, J.; Gu, Y.; Chen, L. In vitro experiment of composite nanofibrous periosteum to promote vascularization and osteogenic mineralization. Chin. J. Tissue Eng. Res. 2023, 27, 4028–4037. [Google Scholar] [CrossRef]
- Kosaras, B.; Jakubowski, M.; Kainz, V.; Burstein, R. Sensory innervation of the calvarial bones of the mouse. J. Comp. Neurol. 2009, 515, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, D.; Xu, C.; Li, H.; Caron, K.M.; Frenette, P.S. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 2021, 589, 591–596. [Google Scholar] [CrossRef]
- Wan, Q.-Q.; Qin, W.-P.; Ma, Y.-X.; Shen, M.-J.; Li, J.; Zhang, Z.-B.; Chen, J.-H.; Tay, F.R.; Niu, L.-N.; Jiao, K. Crosstalk between Bone and Nerves within Bone. Adv. Sci. 2021, 8, 2003390. [Google Scholar] [CrossRef]
- Su, Y.; Zeng, L.; Deng, R.; Ye, B.; Tang, S.; Xiong, Z.; Sun, T.; Ding, Q.; Su, W.; Jing, X.; et al. Endogenous Electric Field-Coupled PD@BP Biomimetic Periosteum Promotes Bone Regeneration through Sensory Nerve via Fanconi Anemia Signaling Pathway. Adv. Healthc. Mater. 2023, 12, e2203027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Li, L.; Ouyang, J.; Wang, T.; Chen, J.; Hu, X.; Ao, Y.; Qin, D.; Zhang, L.; et al. Bioinspired Mild Photothermal Effect-Reinforced Multifunctional Fiber Scaffolds Promote Bone Regeneration. ACS Nano 2023, 17, 6466–6479. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, N.; Feng, L.; Zhao, M.; Wu, P.; Chai, Y.; Liu, J.; Zhu, P.; Guo, R. Multifunctional electrospun asymmetric wettable membrane containing black phosphorus/Rg1 for enhancing infected wound healing. Bioeng. Transl. Med. 2022, 7, e10274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, C.; Liu, Y.; Liu, Z.; Li, J.; Wang, Z.; Han, X. All-in-one bioactive properties of photothermal nanofibers for accelerating diabetic wound healing. Biomaterials 2023, 295, 122029. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Liao, Q.; Zhao, Y.; Huang, H.; Gao, A.; Zhang, W.; Gao, X.; Wei, W.; Guan, M.; Chu, P.K.; et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials 2019, 193, 1–11. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Tan, Y.; Wu, Y.Q. Progress of microspheres in bone tissue engineering. J. Tissue Eng. Reconstr. Surg. 2019, 15, 368–372. [Google Scholar]
- Zhang, Z.; Li, L.; Sun, D.; Wang, M.; Shi, J.; Yang, D.; Wang, L.; Zou, S. Preparation and properties of chitosan-based microspheres by spray drying. Food Sci. Nutr. 2020, 8, 1933–1941. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Yang, J. The Impact of Dexmedetomidine-loaded Nano-microsphere Combined with Percutaneous Acupoint Electrical Stimulation on the Postoperative Cognitive Function of Elderly Patients with Hip Fracture. Cell. Mol. Biol. 2022, 68, 77–85. [Google Scholar] [CrossRef]
- Wang, X.; Shao, J.; Abd El Raouf, M.; Xie, H.; Huang, H.; Wang, H.; Chu, P.K.; Yu, X.-F.; Yang, Y.; AbdEl-Aal, A.M.; et al. Near-infrared light-triggered drug delivery system based on black phosphorus for in vivo bone regeneration. Biomaterials 2018, 179, 164–174. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Zhang, H.; Chen, C.; Zhang, D.; Zhao, Y. Protein-Based Hybrid Responsive Microparticles for Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 18413–18422. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, L.; Luo, Y.; Cai, Z.; Zeng, H.; Wang, T.; Liu, Z.; Chen, Y.; Sheng, X.; Mandlate, A.E.D.G.; et al. Charge-Driven Self-Assembled Microspheres Hydrogel Scaffolds for Combined Drug Delivery and Photothermal Therapy of Diabetic Wounds. Adv. Funct. Mater. 2023, 33, 2214036. [Google Scholar] [CrossRef]
- Song, J.E.; Jun, S.-H.; Park, S.-G.; Kang, N.-G. A Semi-Dissolving Microneedle Patch Incorporating TEMPO-Oxidized Bacterial Cellulose Nanofibers for Enhanced Transdermal Delivery. Polymers 2020, 12, 1873. [Google Scholar] [CrossRef]
- van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control Release Off. J. Control Release Soc. 2012, 161, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Saeed AL-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Pruettijarai, U.; Meephansan, J.; Prapapan, O.; Pureesrisak, P.; Sirithanabadeekul, P.; Tantisantisom, K.; Thongma, S.; Rayanasukha, Y.; Adulyaritthikul, P.; Khanchaitit, P. Efficacy of a novel microneedle patch for rejuvenation of the nasolabial fold. Skin Res. Technol. 2022, 28, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Pillay, V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control Release Off. J. Control Release Soc. 2014, 185, 130–138. [Google Scholar] [CrossRef]
- Yang, H.; Kim, S.; Jang, M.; Kim, H.; Lee, S.; Kim, Y.; Eom, Y.A.; Kang, G.; Chiang, L.; Baek, J.H.; et al. Two-phase delivery using a horse oil and adenosine-loaded dissolving microneedle patch for skin barrier restoration, moisturization, and wrinkle improvement. J. Cosmet. Dermatol. 2019, 18, 936–943. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Liu, Y.; Sun, L.; Sun, L.; Zhao, Y. Black Phosphorus-Loaded Separable Microneedles as Responsive Oxygen Delivery Carriers for Wound Healing. ACS Nano 2020, 14, 5901–5908. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, X.; Nie, M.; Xu, Y.; Wang, Y.; Shang, L.; Zhao, Y.; Zhao, Y. Photothermal Responsive Microspheres-Triggered Separable Microneedles for Versatile Drug Delivery. Adv. Funct. Mater. 2022, 32, 2110746. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, Y.; Liu, R.; Zhao, Y. Globefish-Inspired Balloon Catheter with Intelligent Microneedle Coating for Endovascular Drug Delivery. Adv. Sci. 2022, 9, 2204497. [Google Scholar] [CrossRef]
- Luan, X.; Zhang, X.; Nie, M.; Zhao, Y. Traditional Chinese Medicine Integrated Responsive Microneedles for Systemic Sclerosis Treatment. Research 2023, 6, 0141. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, C.; Wang, M.; Zhang, W.; Xu, B.; Yan, X.; Xin, H.; Wang, X. Black phosphorus modified soluble microneedle patch for painless, effective and accurate body slimming. Appl. Mater. Today 2020, 19, 100577. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, H.; Gao, X.; Zheng, X.; Chen, H.; Li, H.; Peng, J.; Liang, W.; Wang, W.; Qiu, Z.; et al. Bone-Targeting Liposome-Encapsulated Salvianic Acid A Improves Nonunion Healing Through the Regulation of HDAC3-Mediated Endochondral Ossification. Drug Des. Devel. Ther. 2020, 14, 3519–3533. [Google Scholar] [CrossRef]
- Kwon, S.; Yang, J.H.; Shin, J.; Park, K.; Huh, C.; Na, J. Efficacy of liposome-encapsulated 4-n-butylresorcinol and resveratrol cream in the treatment of melasma. J. Cosmet. Dermatol. 2020, 19, 891–895. [Google Scholar] [CrossRef]
- Shakouri, A.; Adljouy, N.; Balkani, S.; Mohamadi, M.; Hamishehkar, H.; Abdolalizadeh, J.; Kazem Shakouri, S. Effectiveness of topical gel of medical leech (Hirudo medicinalis) saliva extract on patients with knee osteoarthritis: A randomized clinical trial. Complement. Ther. Clin. Pract. 2018, 31, 352–359. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wang, J.; Wang, Y.; Chen, A.; Wang, C.; Mo, W.; Li, Y.; Yuan, Q.; Zhang, Y. Photon-Responsive Antibacterial Nanoplatform for Synergistic Photothermal-/Pharmaco-Therapy of Skin Infection. ACS Appl. Mater. Interfaces 2019, 11, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fu, Q.; Ye, J.; Ge, X.; Wang, J.; Song, J.; Yang, H. Ag+-Coupled Black Phosphorus Vesicles with Emerging NIR-II Photoacoustic Imaging Performance for Cancer Immune-Dynamic Therapy and Fast Wound Healing. Angew. Chem.-Int. Ed. 2020, 59, 22202–22209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Zhang, L.; Zhu, C.; Wang, J.; Li, Y.; Wang, Y.; Wang, C.; Zhang, Y.; Yuan, Q. Bioinspired extracellular vesicles embedded with black phosphorus for molecular recognition-guided biomineralization. Nat. Commun. 2019, 10, 2829. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Song, W.; Ma, J.; Wang, N. Macrophage-derived hybrid exosome-mimic nanovesicles loaded with black phosphorus for multimodal rheumatoid arthritis therapy. Biomater. Sci. 2022, 10, 6731–6739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wan, Y.; Xie, H.; Mu, Y.; Du, P.; Wang, D.; Wu, X.; Ji, H.; Wan, L. Degradation Chemistry and Stabilization of Exfoliated Few-Layer Black Phosphorus in Water. J. Am. Chem. Soc. 2018, 140, 7561–7567. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Wu, Z.; Han, Y.; Xu, S.; Wang, L.; Ye, W.; Han, T.; He, Y.; Cai, Y.; et al. High-quality sandwiched black phosphorus heterostructure and its quantum oscillations. Nat. Commun. 2015, 6, 7315. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Wang, L.; Yang, Z.; Wang, H.; Wang, Z.; Cui, Y. Black Phosphorus-Based Drug Nanocarrier for Targeted and Synergetic Chemophotothermal Therapy of Acute Lymphoblastic Leukemia. ACS Appl. Mater. Interfaces 2019, 11, 5896–5902. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, K.; Li, Z.; Wang, C.; Yu, M.; Li, Z.; Li, Z. Black Phosphorus Nanosheets Passivation Using a Tripeptide. Small 2018, 14, e1801701. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Hu, K.; Feng, Y.; Chen, S.; Yang, X.; Fong-Chuen Loo, J.; Zhang, H.; Yin, F.; Li, Z. Surface Coordination of Black Phosphorus with Modified Cisplatin. Bioconjug. Chem. 2019, 30, 1658–1664. [Google Scholar] [CrossRef]
- Yang, B.; Wan, B.; Zhou, Q.; Wang, Y.; Hu, W.; Lv, W.; Chen, Q.; Zeng, Z.; Wen, F.; Xiang, J.; et al. Te-Doped Black Phosphorus Field-Effect Transistors. Adv. Mater. 2016, 28, 9408–9415. [Google Scholar] [CrossRef]
- Zhou, B.; Kang, W.; Jiang, H.; Yang, H.; Li, Z.; Lv, Z.; Xu, Z.; Ning, C.; Wang, H.; Xie, S. Preparation and Crosslinking Mechanism of Delayed Swelling Double-Crosslinking Nano Polymer Gel Microsphere for Anti-CO2 Gas Channeling. Pet. Sci. Eng. 2022, 219, 111–112. [Google Scholar] [CrossRef]
- Huang, L.Y.; Li, W.; Du, N.; Lu, H.Q.; Meng, L.D.; Huang, K.Y.; Li, K. Preparation of quaternary ammo-nium magnetic chitosan microspheres and their application for Congo red adsorption. Carbohydr. Polym. 2022, 297, 119995. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. Biomedecine Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, W.; Zhang, R.; Wang, Z.; Du, H.; Zhao, Y.; Shi, B.; Wang, Y.; Wang, X.; Wang, P. Advances in the Application of Black Phosphorus-Based Composite Biomedical Materials in the Field of Tissue Engineering. Pharmaceuticals 2024, 17, 242. https://0-doi-org.brum.beds.ac.uk/10.3390/ph17020242
Qi W, Zhang R, Wang Z, Du H, Zhao Y, Shi B, Wang Y, Wang X, Wang P. Advances in the Application of Black Phosphorus-Based Composite Biomedical Materials in the Field of Tissue Engineering. Pharmaceuticals. 2024; 17(2):242. https://0-doi-org.brum.beds.ac.uk/10.3390/ph17020242
Chicago/Turabian StyleQi, Wanying, Ru Zhang, Zaishang Wang, Haitao Du, Yiwu Zhao, Bin Shi, Yi Wang, Xin Wang, and Ping Wang. 2024. "Advances in the Application of Black Phosphorus-Based Composite Biomedical Materials in the Field of Tissue Engineering" Pharmaceuticals 17, no. 2: 242. https://0-doi-org.brum.beds.ac.uk/10.3390/ph17020242