1. Introduction
Positron emission tomography (PET) is a powerful tool that offers insights into the molecular and physiological processes underlying various diseases [
1]. It can provide diagnostic information about the homeostatic system without altering its function [
2]. Additionally, various types of information can be collected using PET, including the staging of diseases, the correct selection of pharmacological or radionuclide therapies, responses to treatments and radiotherapy planning, and correct theragnostic approaches [
3,
4]. Additionally, it has been utilized as a tool for development in drug discovery, even in different disciplines, such as research and clinical trials [
5].
Currently, PET radiotracers are produced in centralized, advanced nuclear medicine laboratories and then distributed to nearby clinics. However, many clinics have limited access to radiotracers to meet the immediate needs of their patients, and some specialized tracers may not be accessible due to the short half-life of radionuclides (Carbon-11; 20.34 min) [
6,
7]. The need for advanced, automated, and efficient radiotracer production has driven the development of innovative microfluidic techniques, offering promising solutions to overcome the existing challenges and fill the gap [
8]. Microfluidic techniques offer significant advantages by enabling real-time and patient-specific radiotracer production such as the dose-on-demand approach [
9]. Optimizing precursor quantities and establishing automated microfluidic synthesis protocols using the microfluidic cassette are crucial steps toward realizing the full potential of these batch-type microfluidic techniques to assess their suitability for dose-on-demand production.
11C-based radiopharmaceuticals are valuable for clinical applications because of their ability to provide additional diagnostic information about oncological and neurodegenerative disorders [
5,
10,
11]. [
11C]methyl iodide ([
11C]CH
3I) is used as the radioactive precursor in these radiotracer syntheses. [
11C]choline ([
11C]CHL) is used to evaluate suspected biochemically recurrent prostate cancer [
12], whereas L-[
11C]methionine ([
11C]MET) has shown its potential in the clinic and research as an essential amino acid carrier for multiple metabolic pathways [
13]. It has been used to determine staging, assess prognosis, and evaluate the response to therapy in multiple myeloma [
14] and brain tumors [
15].
The syntheses of [
11C]MET and [
11C]CHL using [
11C]CH
3I have been established [
16,
17,
18,
19]. However, conventional synthesizers are not cost-effective and require high precursor amounts [
20,
21,
22]. Improvements in these requirements necessitate technological advancements to replace the existing synthesizers with upgraded models. This involves integrating state-of-the-art microfluidic techniques into radiosynthesizers to enhance their practicality in preclinical and clinical applications. Therefore, we explored a batch-type iMiDEV
TM microfluidic radiosynthesizer’s ability to produce different radiotracers and their adaptability to routine production using the same module [
23,
24,
25]. The iMiDEV
TM module demonstrated that using 3 to 5 times less precursor allowed it to produce a comparable radiochemical yield to conventional modules [
24,
25,
26,
27,
28].
In this study, we aimed to synthesize [11C]MET and [11C]CHL using a microfluidic cassette-based iMiDEVTM, a fully automated radiochemistry synthesis module, to assess compatibility in a single-dose production. We focused on optimizing the precursor amount for both radiotracers and explored their synthesis in microfluidic cassettes.
2. Results
The application of the microfluidic cassette-based iMiDEV™ module under optimized experimental conditions resulted in promising outcomes for synthesizing [
11C]MET and [
11C]CHL. After the optimization of the parameters, such as the quantities of precursor and radioactivity, the precursor loading conditions, and the microfluidic cassette, the fully automated syntheses were performed during the validation runs. The starting activity of [
11C]CH
4 was 23 GBq and 55 GBq for a 2 min and 5 min beam, respectively. [
11C]CH
3I was 5.5 GBq (5 min beam; 35µA) for both tracers. The precursor amount was optimized for [
11C]MET to 400 µg. The radiochemical yield (RCY) was 84 ± 4%, and the synthesis time was 18 ± 1 min (
n = 3). Similarly, the optimized precursor volume for [
11C]choline was 25 µL, and the RCY was 66 ± 2%. The synthesis time was 24 ± 1 min (
n = 3). The total product volumes of [
11C]MET and [
11C]CHL were 7.8 and 7.5 mL, respectively. The details of the RCYs of [
11C]MET and [
11C]CHL are summarized in
Table 1.
The quality control (QC) results for [
11C]MET and [
11C]CHL demonstrated adherence to the acceptance criteria, ensuring the reliability and suitability of the synthesized radiotracers for clinical applications. High-performance liquid chromatography (HPLC) analysis confirmed that the radiochemical purities were >96.0% for [
11C]MET and >99.0% for [
11C]CHL. The HPLC chromatograms for both radiotracers are provided in
Supplementary Materials, SM (Figures S1 and S2). The stability of the products was analyzed after 90 min, and the purity was >95% for [
11C]MET and >99% for [
11C]CHL. These comprehensive QC assessments highlight the precision and consistency achieved in synthesizing [
11C]MET and [
11C]CHL, thus reinforcing their viability for clinical use. A comprehensive overview of all the QC test results is presented in
Table 2.
3. Discussion
We are evaluating the implementation of the automated on-demand single-dose production of radiotracers tailored to clinic requirements. With a library of clinically relevant radiotracers, our objective is to supply these tracers to the clinic as needed to meet patient demands. In this study, we investigated the suitability of two of these tracers for on-demand single-dose production. The complete optimization of [
11C]MET and [
11C]CHL synthesis was performed using a microfluidic cassette. To do so, a new microfluidic cassette was used for each optimization run. Different types of resins (filled on R4) were tested for both radiotracers because of their unique chemistry. The microfluidic cassette with reagents was inserted on the iMiDEV
TM module, and the precursor was loaded on R4. Then, [
11C]CH
3I was trapped on R4, and the reaction was performed. Later, the product was extracted from R4 into a sterile product vial. The synthesis of [
11C]MET and [
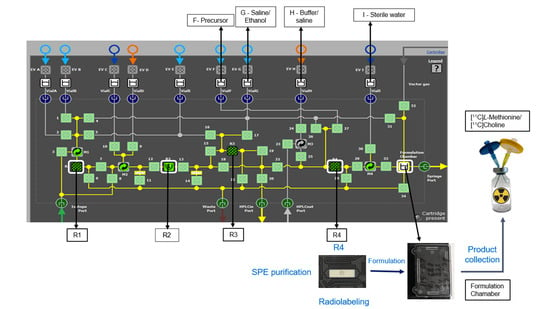
11C]CHL involved the exploration of the microfluidic cassette, particularly reactor 4 (R4) (
Figure 1), and optimization of the precursor amount (L-homocysteine thiolactone and dimethylaminoethanol), loading pressure, and time. The synthesis process is comprehensively automated and does not require any tube handling or specific know-how (including precursor loading, radiolabeling, SPE purification, or formulation). A summary of the optimized conditions for radiosynthesis ([
11C]MET and [
11C]CHL) on R4 in the microfluidic fluidic cassette is presented in
Figure 1.
3.1. Microfluidic Cassette
The microfluidic cassette is one of the main factors influencing the optimization of the synthesis process. There are four reactors in the cassette; three of them (R1, R3, and R4) are used for on-column reactions at room temperature, while reactor 2 is used for reactions at elevated temperatures. In this study, only R4 was chosen to strategically minimize precursor contamination and decrease the overall synthesis time. R1 and R3 have a 50 µL capacity, while R4’s is 200 µL. Reactor 4 was investigated for both [11C]MET and [11C]CHL syntheses using HLB, C18, and CM resins. The back pressure at R4 and the precursor loading pressure and time were optimized to enhance the RCY. The back pressure on R4 is pivotal for precursor loading and thus for [11C]CH3I trapping. The bead density can significantly influence the reaction kinetics, thereby affecting the overall yield.
In the initial tests of [11C]MET synthesis, cassettes filled with C18 and HLB with back pressures between 800 and 870 mbar were chosen, and the precursor loading pressure was fixed at 200 mbar for 35 s. The radiochemical yield fluctuated between 50 and 86% (n = 6). The precursor loading on R4 with a fixed pressure and time was carefully monitored to observe the difference between the yields when using cassettes with back pressures of 800 and 870 mbar. Thereafter, tests were conducted by extending the precursor loading time to 50 s while maintaining a cassette back pressure of 853–877 mbar. However, this alteration did not yield stable results (RCY 39–72%; n = 5). Surprisingly, this adjustment did not improve the radiochemical yield; the RCY was lower than that of the previous method. These fluctuations and lower yields were due to the poor trapping of [11C]CH3I, and the poor precursor dispersion (high probability) on R4 could be explained by the high back pressure and also the increased time, which may have pushed the precursor out of R4 (although there is a negligible chance of this). After these tests, we determined that the density of the beads within them played a pivotal role in the synthesis process. A comparison of the yields also revealed substantial differences, highlighting the importance of cassette selection for synthesis optimization.
We selected only the HLB resin (60 µm) cassettes with similar back pressures (850–870 mbar) for further investigation. Furthermore, we optimized the pressure and time for precursor loading. This aspect is crucial because it directly affects the radiochemical yield. The precursor loading pressure and time were 200 mbar and 30 s, respectively. Under these conditions, the radiochemical yield was stable (RCY 83 ± 3%; n = 5).
For [11C]CHL, CM resin was used because of its suitable properties of high selectivity and sensitivity to strongly basic compounds. We applied the same precursor loading conditions, because ethanol served as the reaction solvent despite the R4 back pressure being approximately 850–900 mbar. However, these conditions did not yield the expected results, resulting in a RCY of 27 ± 15% (n = 3). The higher back pressure at R4, attributed to the CM resin (37–55 µm), can explain this outcome. Subsequently, by optimizing the pressure and time to 150 mbar and 15 s, respectively, and using a lower-volume vial (for the precursor: position F; 300 µL) instead of a 1.5 mL vial, we achieved a consistent and reproducible RCY of 60 ± 7%; n = 7.
The details of the optimized parameters for the syntheses of [
11C]MET and [
11C]CHL are summarized in
Table 3.
3.2. Amount of Precursor and Reaction Time
Different precursor amounts and reaction times were tested to determine their influence on the radiochemical yield. For [
11C]MET synthesis, 1.5 mg of L-homocysteine thiolactone hydrochloride in 500 µL of ethanol were used for the initial test, and the RCY
dc was 76% (
n = 1) and the reaction time was 2 min. After this test, we focused on decreasing the amount of precursor used. We started with 400–500 µg of precursor, based on our previous experience with different tracers [
24]. However, the volume of the precursor vial was maintained at 300 µL because of the larger reactor size (R4 is approximately 200 µL), and the reaction time was increased to 3 min. The radiochemical yield was 62 ± 17% (
n = 3) when the precursor quantity was 400–500 µg. We also used 70% ethanol as the reaction solvent to improve the radiochemical yield; however, there was no increase in the radiochemical yield of 61 ± 8% (
n = 3). When the precursor loading pressure and time were optimized, consistent RCYs were achieved (83 ± 3%;
n = 5). There was no improvement in the yield when the reaction time was increased. The RCYs were between 79 and 88% when the precursor amount was optimized to 400 µg and the loading conditions were optimized (200 mbar, 30 s).
A Further reduction in the precursor amount was performed to investigate the RCY, and the reaction time was maintained at 3 min. The details of the amounts of precursor used with their respective RCYs are summarized in
Table 3. When the precursor amount was decreased from 400 µg to 200 µg, the yield gradually decreased from 83 ± 3% (
n = 5) to 63 ± 6% (
n = 2). When the precursor amount was further reduced to 133 µg, the RCY decreased to 54 ± 4% (
n = 2). These results indicate that lowering the amount of precursor leads to a decrease in the precursor concentration for the reaction, reducing the overall RCY. An illustration of the decline in the RCYs corresponding to variations in the precursor quantity is shown in
Figure 2. The yield decreased linearly when the precursor quantity was decreased from 400 µg to 133 µg. Notably, the precursor concentration was maintained by reducing the precursor volume, because a decreased precursor concentration correlated with lower RCYs. When 133 µg of precursor was used in 100 µL and 200 µL volumes, the RCYs were 54 ± 4% and 34 ± 15%, respectively. The main reason for the lowered yield was the reduction in the precursor’s concentration, resulting in a poor coating of the precursor on the resin within R4. It is important to maintain the homogeneity of the precursor in R4 to achieve consistent RCYs. Finally, the precursor amount was optimized to 400 µg with a 300 µL volume, and validation runs were performed using the same amount of precursor and the same volume, which was five times less than that of conventional radiosynthesizers [
20,
21,
22,
27,
29]. The total synthesis time was 18 min.
For [
11C]CHL, an initial trial was conducted using 100 µL (89 mg) of DMAE in 200 µL of ethanol with a reaction time of 2 min, yielding an RCY
dc of 49% (
n = 1). Subsequent investigations aimed to determine the impact on the RCY by reducing the precursor volume from 100 µL to 12 µL of DMAE. An initial decrease in the yield was observed when the precursor volume was reduced to 50 µL. However, after optimizing the precursor loading conditions, the RCY increased to 60% (
n = 4), surpassing the yield obtained when using 100 µL. RCYs of 58 ± 2% (
n = 4) and 37±7% (
n = 3) were obtained with precursor volumes of 25 µL and 12 µL, respectively, and the RCY decreased as the precursor volume decreased. This phenomenon was attributed to the lower precursor concentration in R4, which increased the unreacted [
11C]CH
3I and was observed during washing with ethanol and water. Notably, no significant difference in yield was observed between 25 and 50 µL. Consequently, the final precursor volume was optimized to 25 µL, which is 2–4 times less than the conventional method [
20,
27].
3.3. Amount of Radioactivity
The effect of the amount of radioactivity used in the synthesis on the radiochemical yield was also investigated. The complete details of the radiochemical yield obtained using different beam times with optimized synthesis conditions are summarized in
Table 4. In the initial exploration of [
11C]MET and [
11C]CHL, a 2 min beam was employed, yielding an approximate [
11C]CH
3I activity of 2.5–3 GBq (
n = 3). Subsequently, the optimization required an extended beam time of 5 min, which increased the [
11C]CH
3I activity (5.5 GBq;
n = 3) to influence the RCY. Interestingly, there was no significant difference in RCY when transitioning from a 2 min to a 5 min beam; the difference was negligible. Specifically, using a 2 min beam for [
11C]MET synthesis yielded 76 ± 7% (
n = 3), while with a 5 min beam the yield slightly increased to 82 ± 3% (
n = 4), indicating minimal variation between the two beam times. Similarly, for [
11C]CHL, the comparison of RCY with beam time revealed a pattern similar to that of [
11C]MET, where the 2 min beam yielded 58 ± 2% (
n = 4) and the 5 min beam yielded 66 ± 2% (
n = 3). An empirical comparison of the yields of [
11C]MET and [
11C]CHL indicated that the amount of radioactivity did not influence the overall RCY.
The optimized synthesis conditions, such as resin type, R4 back pressure, precursor amount, and loading conditions, and RCYs for both [
11C]MET and [
11C]CHL, are summarized in
Table 5.
3.4. Comparison of iMiDEV Module with Conventional Module
The iMiDEV
TM batch-type microfluidic cassette-based synthesis module outperforms conventional synthesizers with its integrated design, reduced reagent volumes, precise control, and improved reproducibility. The iMiDEV
TM system offers an integrated platform with all essential synthesis components, including reactors, reagent reservoirs, and fluidic channels, seamlessly incorporated into a single disposable cassette. This design unlike conventional synthesizers, which typically entail bulkier components and manual intervention for cassette setup, necessitating more extensive setup and space requirements. Additionally, the iMiDEV
TM module employs significantly smaller volumes of reagents (100 µL) and precursors (4–5 times less than conventional modules), resulting in reduced consumption, cost-effectiveness, and minimized waste generation compared to conventional methods. The microfluidic nature of the iMiDEV
TM system allows for precise control over the reaction parameters, resulting in the improved reproducibility and reliability of tracer synthesis. While these significant advancements make iMiDEV
TM superior to conventional synthesis modules, it is important to acknowledge that while the iMiDEV
TM module represents a substantial advancement in microfluidic technology, it may not fulfill all the requirements demanded of the current radiotracers production. Nevertheless, ongoing advancements in microfluidic engineering, coupled with the modular and adaptable design of the iMiDEV
TM system, hold promise for overcoming current limitations and further enhancing the efficiency and versatility of radiotracer production, and are promising for future movement towards dose-on-demand production. Some of the other advantages of the iMiDEV
TM module over conventional modules are summarized in
Table 6.
3.5. Study Limitations and Future Perspectives
This research contributes to the PET radiosynthesis methodology by optimizing the synthesis procedures for [11C]MET and [11C]CHL using a batch-type microfluidic iMiDEV™ synthesis setup. The synthesis model introduced in this study addresses the critical challenges in patient-centric radiotracer production and bolsters on-demand single dose production. While these accomplishments are noteworthy, it is essential to recognize that there are certain limitations, including the study’s focus on specific tracers. Future research should extend beyond [11C]MET and [11C]CHL, encompassing a broader spectrum of radiopharmaceuticals. This expansion aims to enhance the applicability of the dose-on-demand synthesis approach, while concurrently developing synthesis methods that minimize the need for extensive quality control tests. Ongoing efforts to refine microfluidic cassette parameters and explore novel resins will further contribute to the versatility and efficiency of this synthesis method. Commitment to continuous innovation and refinement remains pivotal for advancing PET imaging methodologies.
4. Materials and Methods
[11C]methane ([11C]CH4) was produced using a PET Trace 16.4 MeV Cyclotron from General Electric, Uppsala, Sweden. The microfluidic cassettes and the iMiDEVTM radiosynthesizer were supplied by PMB-Alcen, France. Ethanol (99.5%) was procured from Kiilto Clean AB (Malmö, Sweden). Water (18 MOhm) was obtained using an in-house Milli-Q water purification system (Merck Millipore, Germany). Sterile water and sodium chloride (NaCl 0.9%) were purchased from B Braun, Melsungen, Germany. Sterile filters (Millex GV, 0.22 µm, 33 mm) and vent filters (Millex FG, 0.2 µm, 25 mm) were purchased from Merck Millipore (Carrigtwohill, Ireland). Dimethylaminoethanol (DMAE), L-methionine, and L-homocysteine thiolactone hydrochloride were purchased from Sigma-Aldrich (Darmstadt, Germany). Sodium hydroxide (NaOH), methanol (CH3OH), potassium dihydrogen phosphate (KH2PO4), sodium dihydrogen phosphate (NaH2PO4), and MQuant pH indicator strips were obtained from Merck KGaA (Darmstadt, Germany). Wheaton® W986212NG NextGen™ V Vial® 0.3 mL Clear Glass High Recovery Vials used in syntheses were supplied by Wheaton, USA. Additionally, 1.5 mL vials (V-shaped bottom), inserter vials (300 µL), and aluminum seals with septa (11 mm) were ordered from Thermo Scientific (Langerwehe, Germany). Glass vials (4 and 15 mL) were acquired from the Nordic Pack (Nykvarn, Sweden). Sterile vacuum vials (15 mL) were procured from Huyai Isotopes Co. (Suzhou, China).
4.1. Radiosynthesis of L-[11C]Methionine and [11C]Choline
4.1.1. Preparation of [11C]Methyl Iodide ([11C]CH3I)
[
11C]CH
4 was produced from a methane target via the
14N(p, α)
11C nuclear reaction in a cyclotron. The target was filled with nitrogen gas mixed with 10% hydrogen and bombarded for 2–5 min at 35 µA. [
11C]CH
3I was synthesized using the TracerMaker module with in-target-produced [
11C]CH
4 as previously reported [
30]. After the production of [
11C]CH
3I (~3–5.5 GBq), it was transferred through a separate line directly connected to the iMiDEV™ radiosynthesizer. The flow rate of [
11C]CH
3I was set at 8 mL/min [
24].
4.1.2. Automated Radiosynthesis
iMiDEV™ is a batch-type microfluidic cassette-based radiosynthesizer that produces radiotracers at room and/or elevated temperatures [
23,
24,
25]. This study used a single-use microfluidic cassette with suitable resins for different radiotracers. A picture of the microfluidic cassette with the vial position utilized in this study is shown in
Figure 3. The entire synthesis process, including radiolabeling, solid-phase extraction (SPE) purification, and formulation, was integrated within the microfluidic cassette. All the synthesis steps were performed in auto mode without any manual intervention. A complete overview of the iMiDEV
TM supervision software is provided in the
Supplementary Material (Figure S3). The iMiDEV™ radiosynthesizer is part of the iMiGiNE™ automated radiopharmaceutical production system.
All reagents used for the [
11C]MET and [
11C]CHL syntheses and their respective vial positions are summarized in
Table 7, and the reaction schematics are provided in the
Supplementary Material (Schemes S1 and S2). The procedure for back pressure measurement (flow resistance measurement) in the reactors (R1, R3, and R4) and the bead filling of the reactors was described in our previous publication [
24]. The RCY was calculated by dividing the starting activity by the final obtained product activity. All the yields are decay-corrected unless otherwise mentioned.
4.1.3. [11C]Methionine
The production of [11C]MET involved using the R4 chamber in the microfluidic cassette for the reaction and purification. The HLB (hydrophilic-lipophilic) beads facilitated the trapping of radioactivity in the R4 chamber, and the reaction was performed. The final product was eluted and diluted in the formulation chamber before being transferred to a sterile vial via a sterile filter and through an extraction valve.
For [
11C]MET synthesis, L-homocysteine thiolactone (precursor) was mixed with 99.5% ethanol and 5 M sodium hydroxide. The mixture was vortexed for 3 min before loading into a microfluidic cassette at position F. At position H, 2 mL of 50 mM NaH
2PO
4 phosphate buffer and 6 mL of 0.9% NaCl saline was placed at position G. After all the reagents were loaded, the cassette was placed in the synthesis box, clamped, and pressurized prior to starting the synthesis. Synthesis was initiated by transferring the precursor from vial F to R4 by opening microfluidic valves (MFVs) 27 and 28 (
Figure S3; SM) at a pressure of 200 mbar for 30 s. Once [
11C]CH
3I reached the detectors of R2 from the TracerMaker module through opened MFVs 8, 12, 13, 18, and 20 (
Figure S3; SM), radioactivity was channeled towards the R4 chamber by closing MFV 20 and opening MFVs 22 and 28 (
Figure S3; SM). After the maximum radioactivity was trapped in R4 (
Figure 4), MFV 28 was closed, and the reaction proceeded for 3 min. Following the reaction, the product was eluted with phosphate buffer (NaH
2PO
4) from vial H into the formulation chamber for further dilution. MFVs 24, 25, 29, 33, and 34 (
Figure S3; SM) were opened at a pressure of 1500 mbar for 90 s. A similar process was repeated with vial G through MFVs 26, 29, 33, and 34 (
Figure S3; SM) for dilution of the product with 0.9% NaCl saline at a pressure of 1500 mbar for 60 s.
After all these synthesis steps, including the radiochemical reaction, SPE purification, and formulation, the final product was transferred to a sterile 15 mL product vial through a 0.22 µm sterile filter. The radiosensor data of the complete synthesis of [
11C]MET and [
11C]CHL are provided in
Figure 4.
4.1.4. [11C]Choline
The synthesis was initiated by transferring the precursor from vial F to R4 (filled with CM resin), which was accomplished by opening the valves MFVs 27, 28 (
Figure S3; SM) under a pressure of 150 mbar for 15 s. Once [
11C]CH
3I reached the detector of R2 from the TracerMaker module via the opened MFVs 8, 12, 13, 18, and 20 (
Figure S3; SM), radioactivity was directed toward the R4 chamber valve by closing MFV 20 and opening MFV 22 and MFV 28. After trapping the maximum radioactivity in R4, MFV 28 was closed, and the reaction proceeded for 5 min. Following the reaction, R4 was washed with 3 mL of ethanol and sterile water and then eluted with 8 mL saline into the formulation chamber. MFVs 24, 25, 29, 33, and 34 (
Figure S3; SM) were opened under a pressure of 1950 mbar for 120 s. After the synthesis steps were completed, including the radiochemical reaction, solid-phase extraction (SPE) purification, and formulation, the final product was transferred to a sterile 15 mL product vial through a 0.22 µm sterile filter.
The details of the whole synthesis preparation for [
11C]MET and [
11C]CHL, from the start to the end of the synthesis, are summarized in
Table 8.
5. Conclusions
In conclusion, this study successfully optimized the synthesis of [11C]MET and [11C]CHL using a microfluidic cassette-based iMiDEV™ module, demonstrating its robustness for on-demand single-dose synthesis, which could fulfill a single patient’s requirements or be divided for multiple doses. The meticulous exploration of the microfluidic cassette parameters, including reactor 4, the resin types, and back pressure optimization, demonstrated their pivotal role in ensuring stable and reproducible RCYs. The optimized precursor amount for both [11C]MET and [11C]CHL was 4–5 times less than that of conventional radiosynthesizers, and the obtained final products were sufficient for a single patient dose from a lower starting activity. The impact of the amount of radioactivity on the synthesis revealed that an extended beam time did not significantly alter the yields of either tracer, thereby emphasizing the consistent performance of this synthesis approach utilizing a lower starting activity. Our synthesis process was fully automated, with the iMiDEV™ module seamlessly handling all of the synthesis steps under optimized reaction conditions. This significant step toward complete automation underlines the efficiency and reproducibility of our microfluidic cassette-based synthesis method, offering a promising pathway for a single-patient or multiple-dose production of these critical radiotracers for clinical applications. This systematic exploration and fine-tuning of the microfluidic synthesis process, along with the automated synthesis, offer a technical solution to advance toward fulfilling real-time, patient-specific radiopharmaceutical production. The study yielded [11C]MET of 3233 ± 154 MBq and [11C]CHL of 2368 ± 103 MBq from ~5.5 GBq of [11C]CH3I, confirming the efficacy of the optimized synthesis for clinical applications. Moreover, evaluating and implementing microfluidic modules in routine clinical and preclinical production to produce several other radiotracers is another path open for future research.
Supplementary Materials
The following supporting information can be downloaded at
https://0-www-mdpi-com.brum.beds.ac.uk/article/10.3390/ph17020250/s1, Figure S1: HPLC chromatogram of L-[
11C]methionine (radio and UV detector); Figure S2: HPLC chromatogram of [
11C]choline (radio and refractive index detector); Scheme S1: schematic of L-[
11C]methionine reaction; Scheme S2: schematic of [
11C]choline reaction; Figure S3: an overview of the iMiDEV
TM supervision software (Version supervision 1.0.2.12).
Author Contributions
Conceptualization, H.M., S.N. and L.T.; methodology, H.M., L.B. and S.N.; software, H.M.; validation, H.M.; formal analysis, H.M., S.N. and L.T.; investigation, H.M. and S.M.; resources, S.N., C.H. and L.T.; data curation, H.M.; writing—original draft preparation, H.M.; writing—review and editing, H.M., S.N., C.H., L.T., S.M., L.B. and B.L.; visualization, H.M. and S.N.; supervision, S.N., C.H., L.T. and B.L.; project administration, H.M., S.N. and L.T.; funding acquisition, S.N. and C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to all PET group members from the Psychiatry section, Karolinska Institute, and especially to the quality control team for GC analysis and the radiopharmacy members, Karolinska University Hospital, Stockholm, Sweden.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Basu, S.; Alavi, A. Unparalleled contribution of 18F-FDG PET to medicine over 3 decades. J. Nucl. Med. 2008, 49, 17N–21N, 37N. [Google Scholar]
- Becker, J.; Schwarzenböck, S.M.; Krause, B.J. FDG PET Hybrid Imaging. Recent Results Cancer Res. 2020, 216, 625–667. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Kleist, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.P.; Morgenstern, A.; Schottelius, M.; Fani, M. New Developments in Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2019, 60, 167. [Google Scholar] [CrossRef]
- Suridjan, I.; Comley, R.A.; Rabiner, E.A. The application of positron emission tomography (PET) imaging in CNS drug development. Brain Imaging Behav. 2019, 13, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Yuin, P.; Esterby, M.; van Dam, R.M. Emerging Technologies for Decentralized Production of PET Tracers. In Positron Emission Tomography-Current Clinical and Research Aspects; IntechOpen Limited: London, UK, 2012. [Google Scholar]
- Pascali, G.; Matesic, L. How Far Are We from Dose On Demand of Short-Lived Radiopharmaceuticals? In Perspectives on Nuclear Medicine for Molecular Diagnosis and Integrated Therapy; Springer: Tokyo, Japan, 2016; pp. 79–92. [Google Scholar]
- Mc Veigh, M.; Bellan, L.M. Microfluidic synthesis of radiotracers: Recent developments and commercialization prospects. Lab A Chip 2024. [Google Scholar] [CrossRef] [PubMed]
- Arima, V.; Pascali, G.; Lade, O.; Kretschmer, H.R.; Bernsdorf, I.; Hammond, V.; Watts, P.; De Leonardis, F.; Tarn, M.D.; Pamme, N.; et al. Radiochemistry on chip: Towards dose-on-demand synthesis of PET radiopharmaceuticals. Lab A Chip 2013, 13, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Lodi, F.; Malizia, C.; Castellucci, P.; Cicoria, G.; Fanti, S.; Boschi, S. Synthesis of oncological [11C]radiopharmaceuticals for clinical PET. Nucl. Med. Biol. 2012, 39, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Dollé, F. Carbon-11 and fluorine-18 chemistry devoted to molecular probes for imaging the brain with positron emission tomography. J. Label. Comp. Radiopharm. 2013, 56, 65–67. [Google Scholar] [CrossRef]
- Grkovski, M.; Gharzeddine, K.; Sawan, P.; Schöder, H.; Michaud, L.; Weber, W.A.; Humm, J.L. (11)C-Choline Pharmacokinetics in Recurrent Prostate Cancer. J. Nucl. Med. 2018, 59, 1672–1678. [Google Scholar] [CrossRef]
- Parkhitko, A.A.; Jouandin, P.; Mohr, S.E.; Perrimon, N. Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell 2019, 18, e13034. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Kircher, M.; Da Via, M.; Schreder, M.; Rasche, L.; Kortüm, K.M.; Einsele, H.; Buck, A.K.; Hänscheid, H.; Samnick, S. Comparison of 11C-Choline and 11C-Methionine PET/CT in Multiple Myeloma. Clin. Nucl. Med. 2019, 44, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Kimura, K.; Abe, K.; Sakai, S. (11)C-methionine PET/CT findings in benign brain disease. Jpn. J. Radiol. 2017, 35, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Någren, K.; Halldin, C. Methylation of amide and thiol functions with [11C]methyl triflate, as exemplified by [11C]NMSP[11C]flumazenil and [11C]methionine. J. Label. Compd. Radiopharm. 1998, 41, 831–841. [Google Scholar] [CrossRef]
- Pascali, C.; Bogni, A.; Itawa, R.; Cambiè, M.; Bombardieri, E. [11C]Methylation on a C18 Sep-Pak cartridge: A convenient way to produce [N-methyl-11C]choline. J. Label. Compd. Radiopharm. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Ohtani, T.; Kurihara, H.; Ishiuchi, S.; Saito, N.; Oriuchi, N.; Inoue, T.; Sasaki, T. Brain tumour imaging with carbon-11 choline: Comparison with FDG PET and gadolinium-enhanced MR imaging. Eur. J. Nucl. Med. 2001, 28, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Pascali, C.; Bogni, A.; Iwata, R.; Decise, D.; Crippa, F.; Bombardieri, E. High efficiency preparation of L-[S-methyl-11C]methionine by on-column [11C]methylation on C18 Sep-Pak. J. Label. Compd. Radiopharm. 1999, 42, 715–724. [Google Scholar] [CrossRef]
- Wenz, J.; Arndt, F.; Samnick, S. A new concept for the production of (11)C-labelled radiotracers. EJNMMI Radiopharm. Chem. 2022, 7, 6. [Google Scholar] [CrossRef]
- Kilian, K.; Pękal, A.; Juszczyk, J.J. Synthesis of 11C-methionine through gas phase iodination using Synthra MeIPlus synthesis module. Nukleonika 2016, 61, 29–33. [Google Scholar] [CrossRef]
- Woods, M.; Leung, L.; Frantzen, K.; Garrick, J.G.; Zhang, Z.; Zhang, C.; English, W.; Wilson, D.; Bénard, F.; Lin, K.S. Improving the stability of (11)C-labeled L-methionine with ascorbate. EJNMMI Radiopharm. Chem. 2017, 2, 13. [Google Scholar] [CrossRef]
- Ovdiichuk, O.; Mallapura, H.; Pineda, F.; Hourtané, V.; Långström, B.; Halldin, C.; Nag, S.; Maskali, F.; Karcher, G.; Collet, C. Implementation of iMiDEV™, a new fully automated microfluidic platform for radiopharmaceutical production. Lab Chip 2021, 21, 2272–2282. [Google Scholar] [CrossRef]
- Mallapura, H.; Tanguy, L.; Långström, B.; Meunier, L.L.; Halldin, C.; Nag, S. Production of [11C]Carbon Labelled Flumazenil and L-Deprenyl Using the iMiDEV™ Automated Microfluidic Radiosynthesizer. Molecules 2022, 27, 8843. [Google Scholar] [CrossRef] [PubMed]
- Mallapura, H.; Ovdiichuk, O.; Jussing, E.; Thuy, T.A.; Piatkowski, C.; Tanguy, L.; Collet-Defossez, C.; Långström, B.; Halldin, C.; Nag, S. Microfluidic-based production of [68Ga]Ga-FAPI-46 and [68Ga]Ga-DOTA-TOC using the cassette-based iMiDEV™ microfluidic radiosynthesizer. EJNMMI Radiopharm. Chem. 2023, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Jussing, E.; Milton, S.; Samén, E.; Moein, M.M.; Bylund, L.; Axelsson, R.; Siikanen, J.; Tran, T.A. Clinically Applicable Cyclotron-Produced Gallium-68 Gives High-Yield Radiolabeling of DOTA-Based Tracers. Biomolecules 2021, 11, 1118. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Hoareau, R.; Runkle, A.C.; Tluczek, L.J.M.; Hockley, B.G.; Henderson, B.D.; Scott, P.J.H. Highlighting the versatility of the Tracerlab synthesis modules. Part 2: Fully automated production of [11C]-labeled radiopharmaceuticals using a Tracerlab FXC-Pro. J. Label. Compd. Radiopharm. 2011, 54, 819–838. [Google Scholar] [CrossRef]
- Wilson, A.A.; Garcia, A.; Jin, L.; Houle, S. Radiotracer synthesis from [ 11C]-iodomethane: A remarkably simple captive solvent method. Nucl. Med. Biol. 2000, 27, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Giglio, J.; Rosas, G.; Basso, M.; Boné, A.; Savio, E.; Engler, H. An alternative methodology for the determination of the radiochemical purity of 11C-methionine. EJNMMI Radiopharm. Chem. 2018, 3, 15. [Google Scholar] [CrossRef]
- Andersson, J.; Truong, P.; Halldin, C. In-target produced [11C]methane: Increased specific radioactivity. Appl. Radiat. Isot. 2009, 67, 106–110. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).