The Effect of Three Different Strategies Based on Motor Task Performance on Neuromuscular Fatigue in Healthy Men and Men with Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
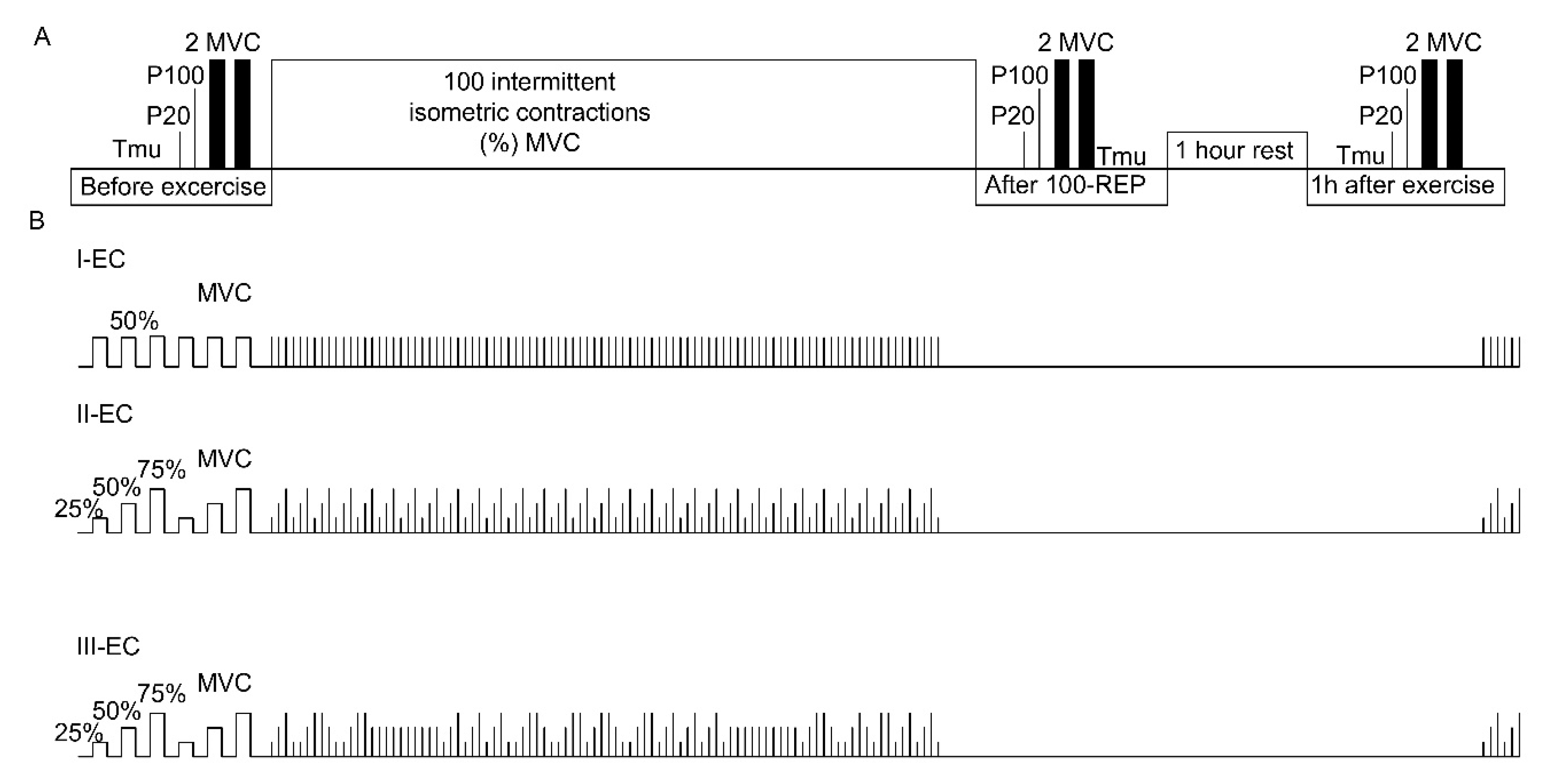
2.3. Experimental Protocol
2.4. The Three IIC ECs
2.5. Measurements
2.6. Isometric Torque and Electrical Stimulation
2.7. Central Activation Ratio (CAR) Measurements
2.8. Muscle Temperature Measurements
2.9. Rating of Perceived Exertion (RPE)
2.10. Accuracy and Motor Variability of IIC Assessment
2.11. Statistical Analyses
3. Results
3.1. Participants’ Characteristics
3.2. Baseline Neuromuscular and Intramuscular Temperature (Tmu) Characteristics
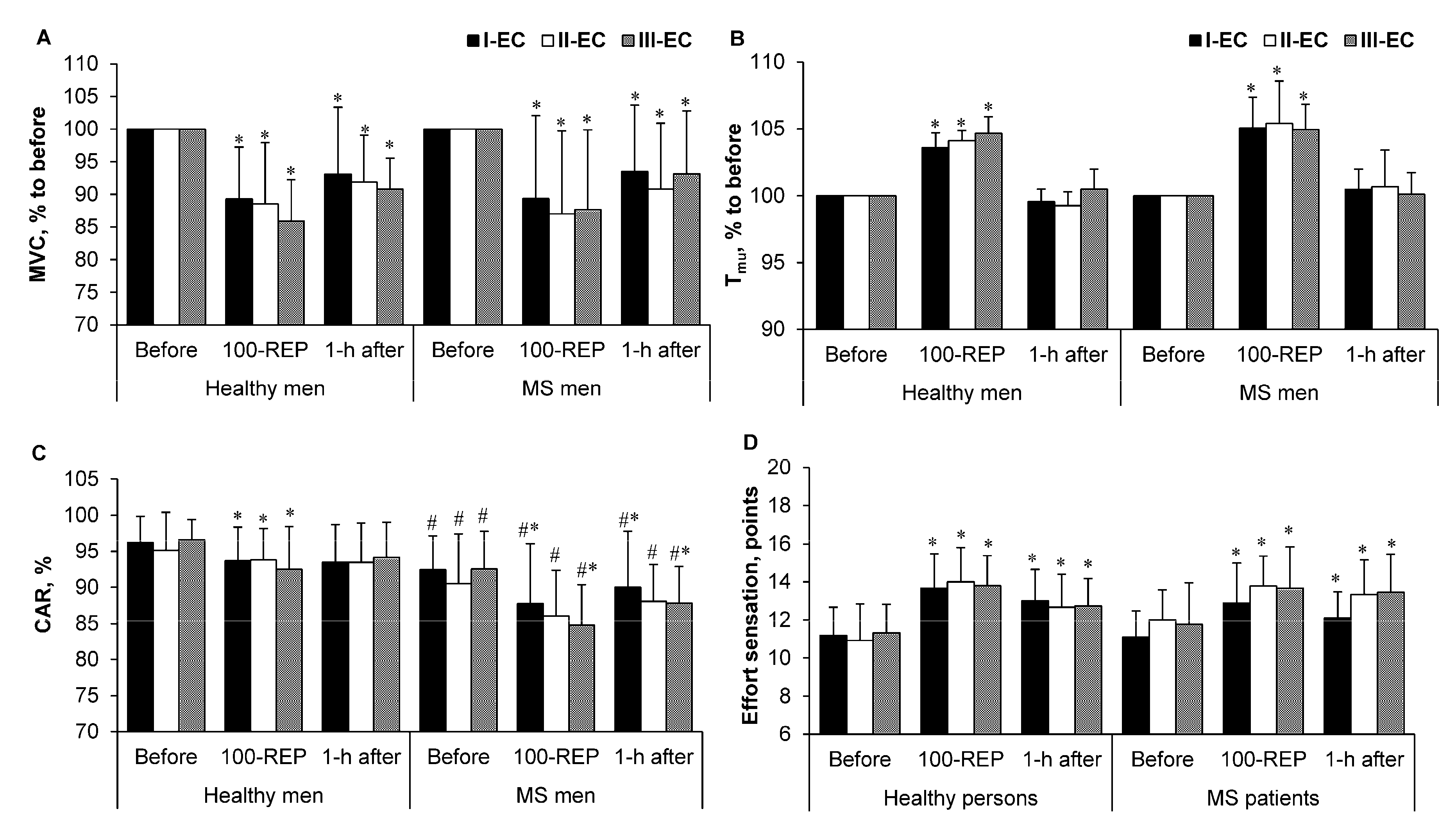
3.3. Maximal Voluntary Contraction (MVC ) Torque, Intramuscular Temperature(Tmu), Central Activation Ratio (CAR) and Rating of Perceived Exertion (RPE)
3.4. Electrically Induced Muscle Torque
3.5. Accuracy and Motor Variability of IIC Tasks
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Brazaitis, M.; Skurvydas, A.; Pukėnas, K.; Daniuseviciūtė, L.; Mickevicienė, D.; Solianik, R. The effect of temperature on amount and structure of motor variability during 2-minute maximum voluntary contraction. Muscle Nerve 2012, 46, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Skurvydas, A.; Brazaitis, M.; Andrejeva, J.; Mickeviciene, D.; Streckis, V. The effect of multiple sclerosis and gender on central and peripheral fatigue during 2-min MVC. Clin. Neurophysiol. 2011, 122, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Skurvydas, A.; Mamkus, G.; Kamandulis, S.; Dudoniene, V.; Valanciene, D.; Westerblad, H. Mechanisms of force depression caused by different types of physical exercise studied by direct electrical stimulation of human quadriceps muscle. Eur. J. Appl. Physiol. 2016, 116, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural contributions to muscle fatigue: From the brain to the muscle and back again. Med. Sci. Sports Exerc. 2016, 48, 2294–2306. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L. The concept of fatigue in multiple sclerosis. J. Neurosci. Nurs. 2008, 40, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Leocani, L.; Colombo, B.; Comi, G. Physiopathology of fatigue in multiple sclerosis. Neurol. Sci. 2008, 29, S241–S243. [Google Scholar] [CrossRef] [PubMed]
- Sheean, G.L.; Murray, N.M.; Rothwell, J.C.; Miller, D.H.; Thompson, A.J. An electrophysiological study of the mechanism of fatigue in multiple sclerosis. Brain 1997, 120 Pt 2, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Severijns, D.; Zijdewind, I.; Dalgas, U.; Lamers, I.; Lismont, C.; Feys, P. The assessment of motor fatigability in persons with multiple sclerosis: A systematic review. Neurorehabil. Neural Repair 2017, 31, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A.; Miller, R.G. Central fatigue during isometric exercise in amyotrophic lateral sclerosis. Muscle Nerve 2000, 23, 909–914. [Google Scholar] [CrossRef]
- Dalgas, U. Exercise therapy in multiple sclerosis and its effects on function and the brain. Neurodegener. Dis. Manag. 2017, 7, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Sandroff, B.M.; Kwakkel, G.; Dalgas, U.; Feinstein, A.; Heesen, C.; Feys, P.; Thompson, A.J. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017, 16, 848–856. [Google Scholar] [CrossRef]
- Zimmer, P.; Bloch, W.; Schenk, A.; Oberste, M.; Riedel, S.; Kool, J.; Langdon, D.; Dalgas, U.; Kesselring, J.; Bansi, J. High-intensity interval exercise improves cognitive performance and reduces matrix metalloproteinases-2 serum levels in persons with multiple sclerosis: A randomized controlled trial. Mult. Scler. 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dalgas, U.; Stenager, E.; Ingemann-Hansen, T. Multiple sclerosis and physical exercise: Recommendations for the application of resistance-, endurance- and combined training. Mult. Scler. 2008, 14, 35–53. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Dressendorfer, R.H. Exercise and multiple sclerosis. Sports Med. 2004, 34, 1077–1100. [Google Scholar] [CrossRef] [PubMed]
- Kjølhede, T.; Vissing, K.; de Place, L.; Pedersen, B.G.; Ringgaard, S.; Stenager, E.; Petersen, T.; Dalgas, U. Neuromuscular adaptations to long-term progressive resistance training translates to improved functional capacity for people with multiple sclerosis and is maintained at follow-up. Mult. Scler. 2015, 21, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Kyguoliene, L.; Skurvydas, A.; Eimantas, N.; Baranauskienė, N.; Mickeviciene, D.; Urboniene, D.; Cernych, M.; Brazaitis, M. Effect of constant, predictable, and unpredictable motor tasks on motor performance and blood markers of stress. Exp. Brain Res. 2017, 235, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale. Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Simpson, S., Jr.; Tan, H.; Otahal, P.; Taylor, B.; Ponsonby, A.L.; Lucas, R.M.; Blizzard, L.; Valery, P.C.; Lechner-Scott, J.; Shaw, C.; et al. Anxiety, depression and fatigue at 5-year review following CNS demyelination. Acta Neurol. Scand. 2016, 134, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Bernecke, V.; Pukenas, K.; Imbrasiene, D.; Mickeviciene, D.; Baranauskiene, N.; Eimantas, N.; Brazaitis, M. Test-retest cross-reliability of tests to assess neuromuscular function as a multidimensional concept. J. Strength Cond. Res. 2015, 29, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Osu, R.; Burdet, E.; Franklin, D.W.; Milner, T.E.; Kawato, M. Different mechanisms involved in adaptation to stable and unstable dynamics. J. Neurophysiol. 2003, 90, 3255–3269. [Google Scholar] [CrossRef] [PubMed]
- Brazaitis, M.; Eimantas, N.; Daniuseviciute, L.; Vitkauskiene, A.; Paulauskas, H.; Skurvydas, A. Two strategies for the acute response to cold exposure but one strategy for the response to heat stress. Int. J. Hyperth. 2015, 31, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Venckunas, T.; Skurvydas, A.; Brazaitis, M.; Kamandulis, S.; Snieckus, A.; Moran, C.N. Human alpha actinin-3 genotype association with exercise-induced muscle damage and the repeated-bout effect. Appl. Physiol. Nutr. Metab. 2012, 37, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Brazaitis, M.; Skurvydas, A.; Vadopalas, K.; Daniusevičiūtė, L.; Senikienė, Z. The effect of heating and cooling on time course of voluntary and electrically induced muscle force variation. Medicina (Kaunas) 2011, 47, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Sternad, D. Motor learning: Changes in the structure of variability in a redundant task. Adv. Exp. Med. Biol. 2009, 629, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Skurvydas, A.; Zachovajevas, P. Is post-tetanic potentiation, low frequency fatigue (LFF) and pre-contractile depression (PCD) coexistent in intermittent isometric exercises of maximal intensity? Acta Physiol. Scand. Suppl. 1998, 164, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Seitz, L.B.; Haff, G.G. Factors modulating post-activation potentiation of jump, sprint, throw, and upper-body ballistic performances: A systematic review with meta-analysis. Sports Med. 2016, 46, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2014, 363, 978–988. [Google Scholar] [CrossRef]

| Healthy Men (n = 15) | MS Men (n = 9) | |
|---|---|---|
| Age, yr. | 22.47 ± 3.74 | 31.11 ± 8.51 # |
| Height, cm | 182.53 ± 6.77 | 178.67 ± 5.79 |
| Mass, kg | 78.51 ± 8.09 | 73.13 ± 6.72 |
| Body mass index, kg/m2 | 23.58 ± 2.29 | 22.90 ± 1.53 |
| Fat free mass, kg | 12.67 ± 4.34 | 12.42 ± 3.99 |
| Body fat, % | 15.86 ± 4.15 | 16.77 ± 4.46 |
| EDSS, points | 3.1 ± 1.3 |
| MVC (N·m) | CAR (%) | Tmu (°C) | ||
|---|---|---|---|---|
| I-EC | Healthy men | 316.79 ± 44.42 | 96.22 ± 3.65 | 36.9 ± 0.5 |
| MS men | 234.51 ± 39.83 # | 92.44 ± 4.72 # | 36.2 ± 0.5 # | |
| II-EC | Healthy men | 310.83 ± 40.16 | 95.15 ± 5.25 | 36.9 ± 0.4 |
| MS men | 228.84 ± 41.37 # | 90.55 ± 6.87 # | 36.0 ± 1.0 # | |
| III-EC | Healthy men | 320.28 ± 36.40 | 96.57 ± 2.83 | 36.7 ± 0.6 |
| MS men | 231.57 ± 47.88 # | 92.55 ± 5.22 # | 36.3 ± 0.6 # | |
| CV (%) | ||||
|---|---|---|---|---|
| Before Exercise | After 100-REP | 1-h after Exercise | ||
| I-EC | Healthy men | 4.1 ± 1.7 | 3.5 ± 1.5 | 3.3 ± 1.5 |
| MS men | 6.1 ± 3.5 | 3.0 ± 1.9 | 2.9 ± 2.5 | |
| II-EC | Healthy men | 5.6 ± 3.7 | 5.0 ± 2.1 | 3.9 ± 1.6 |
| MS men | 5.1 ± 3.6 | 2.9 ± 1.4 | 4.8 ± 4.8 | |
| III-EC | Healthy men | 6.8 ± 8.3 | 3.4 ± 1.1 | 3.4 ± 2.0 |
| MS men | 3.9 ± 2.6 | 3.3 ± 1.5 | 4.7 ± 3.6 | |
| CE (N·m) | ||||
| I-EC | Healthy men | −4.4 ± 4.9 | −2.1 ± 6.4 | −2.6 ± 5.9 |
| MS men | −5.21 ± 4.01 | −5.07 ± 5.17 | −6.39 ± 5.86 | |
| II-EC | Healthy men | −8.8 ± 6.7 | −3.2 ± 5.8 | −3.9 ± 4.9 |
| MS men | −7.31 ± 5.64 | −5.14 ± 6.92 | −7.44 ± 5.68 | |
| III-EC | Healthy men | −7.4 ± 8.9 | −3.5 ± 5.2 | −6.6 ± 9.9 |
| MS men | −6.87 ± 5.09 | −4.23 ± 4.11 | −7.63 ± 4.27 | |
| AE (N·m) | ||||
| I-EC | Healthy men | 7.1 ± 2.6 | 6.4 ± 1.9 | 6.4 ± 2.9 |
| MS men | 7.70 ± 2.37 | 6.89 ± 2.10 | 7.89 ± 3.74 | |
| II-EC | Healthy men | 10.2 ± 4.6 | 7.0 ± 2.1 | 6.5 ± 2.3 |
| MS men | 7.61 ± 5.42 | 7.49 ± 4.42 | 7.69 ± 5.39 | |
| III-EC | Healthy men | 9.3 ± 8.1 | 6.1 ± 3.6 | 7.4 ± 9.6 |
| MS men | 7.48 ± 4.00 | 5.88 ± 1.94 | 8.14 ± 4.31 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyguolienė, L.; Skurvydas, A.; Eimantas, N.; Baranauskienė, N.; Balnytė, R.; Brazaitis, M. The Effect of Three Different Strategies Based on Motor Task Performance on Neuromuscular Fatigue in Healthy Men and Men with Multiple Sclerosis. Medicina 2018, 54, 33. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54030033
Kyguolienė L, Skurvydas A, Eimantas N, Baranauskienė N, Balnytė R, Brazaitis M. The Effect of Three Different Strategies Based on Motor Task Performance on Neuromuscular Fatigue in Healthy Men and Men with Multiple Sclerosis. Medicina. 2018; 54(3):33. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54030033
Chicago/Turabian StyleKyguolienė, Laura, Albertas Skurvydas, Nerijus Eimantas, Neringa Baranauskienė, Renata Balnytė, and Marius Brazaitis. 2018. "The Effect of Three Different Strategies Based on Motor Task Performance on Neuromuscular Fatigue in Healthy Men and Men with Multiple Sclerosis" Medicina 54, no. 3: 33. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina54030033





