Carbohydrate Antigen 50: Values for Diagnosis and Prognostic Prediction of Intrahepatic Cholangiocarcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. CA50 and CA19-9 Analysis
2.3. Statistical Analysis
3. Results
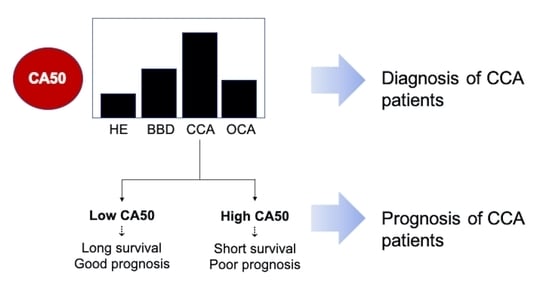
3.1. CA50 Was Elevated in the Sera of CCA Patients and Has a Diagnostic Value for iCCA
3.2. The Increase of Serum CA50 Was Associated with the Severity of Bile Duct Pathology
3.3. High Level of CA50 of iCCA Patients Indicates Poor Prognosis and Shorter Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Razumilava, N.; Gores, G.J. Classification, diagnosis, and management of cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2013, 11, 13Ce4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, C.; Lymp, J.; Angulo, P.; Gores, G.J.; Larusso, N.; Lindor, K.D. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig. Dis. Sci. 2005, 50, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, P.; Enders, F.B.; Halling, K.C.; Lindor, K.D. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology 2008, 48, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.J.; Chen, J.; Liu, W.; Li, J.W.; Jiang, P.; Zhao, X.; Guo, F.; Li, X.W.; Wang, S.G. Application of joint detection of AFP, CA19-9, CA125 and CEA in identification and diagnosis of cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2015, 16, 3451–3455. [Google Scholar] [CrossRef] [Green Version]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Koch, A.; Vucur, M.; Schneider, A.T.; Binnebösel, M.; Ulmer, T.F.; Lurje, G.; Schoening, W. CEA but not CA19-9 is an independent prognostic factor in patients undergoing resection of cholangiocarcinoma. Sci. Rep. 2017, 7, 16975. [Google Scholar] [CrossRef] [Green Version]
- Holmgren, J.; Lindholm, L.; Persson, B.; Lagergård, T.; Nilsson, O.; Svennerholm, L.; Rudenstam, C.M.; Unsgaard, B.; Yngvason, F.; Pettersson, S. Detection by monoclonal antibody of carbohydrate antigen CA 50 in serum of patients with carcinoma. Br. Med. J. 1984, 288, 1479–1482. [Google Scholar] [CrossRef] [Green Version]
- Månsson, J.E.; Fredman, P.; Nilsson, O.; Lindholm, L.; Holmgren, J.; Svennerholm, L. Chemical structure of carcinoma ganglioside antigens defined by monoclonal antibody C-50 and some allied gangliosides of human pancreatic adenocarcinoma. Biochim. Biophys. Acta 1985, 834, 110–117. [Google Scholar] [CrossRef]
- Shan, M.; Tian, Q.; Zhang, L. Serum CA50 levels in patients with cancers and other diseases. Prog. Mol. Biol. Transl. Sci. 2019, 162, 187–198. [Google Scholar]
- Bunworasate, U.; Voravud, N. CA 50: A tumor marker for gastrointestinal malignancies. J. Med. Assoc. Thail. 1995, 78, 255–270. [Google Scholar]
- Haglund, C.; Kuusela, P.; Jalanko, H.; Roberts, P.J. Serum CA 50 as a tumor marker in pancreatic cancer: A comparison with CA 19-9. Int. J. Cancer 1987, 39, 477–481. [Google Scholar] [CrossRef]
- Harmenberg, U.; Wahren, B.; Wiechel, K.L. Tumor markers carbohydrate antigens CA 19-9 and CA-50 and carcinoembryonic antigen in pancreatic cancer and benign diseases of the pancreatobiliary tract. Cancer Res. 1988, 48, 1985–1988. [Google Scholar] [PubMed]
- Haglund, C.; Lindgren, J.; Roberts, P.J.; Nordling, S. Difference in tissue expression of tumour markers CA 19-9 and CA 50 in hepatocellular carcinoma and cholangiocarcinoma. Br. J. Cancer 1991, 63, 386–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Chigusa, M.; Takahashi, H.; Nakamura, J.; Tanaka, H.; Ohno, T. High level of CA19-9, CA50, and CEA-producible human cholangiocarcinoma cell line changes in the secretion ratios in vitro or in vivo. In Vitro Cell. Dev. Biol. Anim. 2000, 36, 104–109. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Detarya, M.; Sawanyawisuth, K.; Aphivatanasiri, C.; Chuangchaiya, S.; Saranaruk, P.; Sukprasert, L.; Silsirivanit, A.; Araki, N.; Wongkham, S.; Wongkham, C. The O-GalNAcylating enzyme GALNT5 mediates carcinogenesis and progression of cholangiocarcinoma via activation of AKT/ERK signaling. Glycobiology 2020, 30, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Juntavee, A.; Sripa, B.; Pugkhem, A.; Khuntikeo, N.; Wongkham, S. Expression of sialyl Lewis(a) relates to poor prognosis in cholangiocarcinoma. World J. Gastroenterol. 2005, 11, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Phoomak, C.; Park, D.; Silsirivanit, A.; Sawanyawisuth, K.; Vaeteewoottacharn, K.; Detarya, M.; Wongkham, C.; Lebrilla, C.B.; Wongkham, S. O-GlcNAc-induced nuclear translocation of hnRNP-K is associated with progression and metastasis of cholangiocarcinoma. Mol. Oncol. 2019, 13, 338–357. [Google Scholar] [CrossRef]
- Silsirivanit, A.; Araki, N.; Wongkham, C.; Vaeteewoottacharn, K.; Pairojkul, C.; Kuwahara, K.; Narimatsu, Y.; Sawaki, H.; Narimatsu, H.; Okada, S. CA-S27: A novel Lewis A associated carbohydrate epitope is diagnostic and prognostic for cholangiocarcinoma. Cancer Sci. 2013, 104, 1278–1284. [Google Scholar] [CrossRef]
- Wattanavises, S.; Silsirivanit, A.; Sawanyawisuth, K.; Cha’on, U.; Waraasawapati, S.; Saentaweesuk, W.; Luang, S.; Chalermwat, C.; Wongkham, C.; Wongkham, S. Increase of MAL-II binding alpha2,3-sialylated glycan is associated with 5-FU resistance and short survival of cholangiocarcinoma patients. Medicina 2019, 55, 761. [Google Scholar] [CrossRef] [Green Version]
- Saentaweesuk, W.; Silsirivanit, A.; Vaeteewoottacharn, K.; Sawanyawisuth, K.; Pairojkul, C.; Cha’on, U.; Indramanee, S.; Pinlaor, S.; Boonmars, T.; Araki, N. Clinical significance of GalNAcylated glycans in cholangiocarcinoma: Values for diagnosis and prognosis. Clin. Chim. Acta 2018, 477, 66–71. [Google Scholar] [CrossRef]
- Silsirivanit, A.; Araki, N.; Wongkham, C.; Pairojkul, C.; Narimatsu, Y.; Kuwahara, K.; Narimatsu, H.; Wongkham, S.; Sakaguchi, N. A novel serum carbohydrate marker on mucin 5AC: Values for diagnostic and prognostic indicators for cholangiocarcinoma. Cancer 2011, 117, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.Y.; Cai, L.; He, X.D.; Liu, W.; Qu, Q. Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Am. Surg. 2010, 76, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Chan-On, W.; Nairismagi, M.L.; Ong, C.K.; Lim, W.K.; Dima, S.; Pairojkul, C.; Lim, K.H.; McPherson, J.R.; Cutcutache, I.; Heng, H.L. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat. Genet. 2013, 45, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Jinawath, N.; Chamgramol, Y.; Furukawa, Y.; Obama, K.; Tsunoda, T.; Sripa, B.; Pairojkul, C.; Nakamura, Y. Comparison of gene expression profiles between Opisthorchis viverrini and non-Opisthorchis viverrini associated human intrahepatic cholangiocarcinoma. Hepatology 2006, 44, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Gu, S.; Wang, P.; Chen, H.; Chen, Z.; Meng, Z.; Liu, L. Pretreatment values of bilirubin and albumin are not prognostic predictors in patients with advanced pancreatic cancer. Cancer Med. 2018, 7, 5943–5951. [Google Scholar] [CrossRef]
- Liu, X.; Cai, H.; Wang, Y. Prognostic significance of tumor markers in T4a gastric cancer. World J. Surg. Oncol. 2012, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- Mittal, A.; Gupta, S.P.; Jha, D.K.; Sathian, B.; Poudel, B. Impact of various tumor markers in prognosis of gastric cancer. A Hospital Based Study from Tertiary Care Hospital of Kathmandu Valley. Asian Pac. J. Cancer Prev. 2013, 14, 1965–1967. [Google Scholar] [CrossRef] [Green Version]


| Index | Group of Subjects | |||
|---|---|---|---|---|
| Healthy (HE) | Benign (BBD) | Other Cancers (OCA) | iCCA | |
| n | 110 | 23 | 33 | 85 |
| CA50 Level (U/mL) | ||||
| Mean | 5.1 | 1195.0 | 156.9 | 8348.0 |
| Median | 4.0 | 15.5 | 4.1 | 197.3 |
| Min | 0.5 | 0.5 | 0.5 | 0.5 |
| Max | 25.6 | 12,460.0 | 2664.0 | 125,000.0 |
| Number of Cases at Cut-Off CA50 = 25.0 U/mL | ||||
| CA50 ≤ 25.0 U/ml | 109 | 12 | 24 | 29 |
| CA50 > 25.0 U/ml | 1 | 11 | 9 | 56 |
| Number of Cases at Cut-Off CA50 = 85.3 U/mL | ||||
| CA50 ≤ 85.3 U/ml | 110 | 19 | 28 | 38 |
| CA50 > 85.3 U/ml | 0 | 4 | 5 | 47 |
| Analyses | Non-CCA vs. iCCA | BBD vs. iCCA | |
|---|---|---|---|
| CA19-9 | CA50 | CA50 | |
| Mann–Whitney U test | |||
| p | <0.001 | <0.001 | 0.039 |
| ROC Analysis | |||
| AUC | 0.797 | 0.806 | 0.641 |
| p | <0.001 | <0.001 | 0.039 |
| Cut-off value | 37 U/mL | 25 U/mL | 85.3 U/mL |
| Sensitivity (%) | 65.9 | 65.9 | 55.3 |
| Specificity (%) | 86.1 | 87.3 | 82.6 |
| Positive predictive value (%) | 70.9 | 72.7 | 92.2 |
| Negative predictive value (%) | 83.1 | 83.3 | 33.3 |
| False positive (%) | 13.9 | 12.7 | 17.4 |
| False negative (%) | 34.1 | 34.1 | 44.7 |
| Accuracy (%) | 79.3 | 80.1 | 61.1 |
| Parameters | n | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| Age (years) | |||||||
| ≤56 | 49 | 1 | - | 1 | - | ||
| >56 | 36 | 1.236 | 0.777–1.965 | 0.370 | 1.506 | 0.908–2.500 | 0.113 |
| Sex | |||||||
| Male | 60 | 1 | - | 1 | - | ||
| Female | 25 | 0.729 | 0.444–1.199 | 0.213 | 0.725 | 0.425–1.235 | 0.236 |
| Histological types | |||||||
| Papillary | 20 | 1 | 1 | - | |||
| Non-papillary | 65 | 1.903 | 1.060–3.418 | 0.031 | 1.963 | 1.036–3.720 | 0.039 |
| Tumor stage | |||||||
| I–III | 7 | 1 | - | - | |||
| IVA | 25 | 1.954 | 0.783–4.881 | 0.151 | 1.801 | 0.691–4.696 | 0.229 |
| IVB | 52 | 2.725 | 1.129–6.577 | 0.026 | 1.744 | 0.676–4.498 | 0.250 |
| CA50 (U/mL) | |||||||
| Low | 43 | 1 | - | - | |||
| Medium | 24 | 1.659 | 0.964–2.854 | 0.068 | 1.447 | 0.689–3.040 | 0.329 |
| High | 46 | 3.629 | 1.961–6.714 | <0.001 | 2.988 | 1.269–7.032 | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luang, S.; Teeravirote, K.; Saentaweesuk, W.; Ma-In, P.; Silsirivanit, A. Carbohydrate Antigen 50: Values for Diagnosis and Prognostic Prediction of Intrahepatic Cholangiocarcinoma. Medicina 2020, 56, 616. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina56110616
Luang S, Teeravirote K, Saentaweesuk W, Ma-In P, Silsirivanit A. Carbohydrate Antigen 50: Values for Diagnosis and Prognostic Prediction of Intrahepatic Cholangiocarcinoma. Medicina. 2020; 56(11):616. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina56110616
Chicago/Turabian StyleLuang, Sukanya, Karuntarat Teeravirote, Waraporn Saentaweesuk, Prasertsri Ma-In, and Atit Silsirivanit. 2020. "Carbohydrate Antigen 50: Values for Diagnosis and Prognostic Prediction of Intrahepatic Cholangiocarcinoma" Medicina 56, no. 11: 616. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina56110616