Screening for Small Molecule Inhibitors of BMP-Induced Osteoblastic Differentiation from Indonesian Marine Invertebrates
Abstract
:1. Introduction
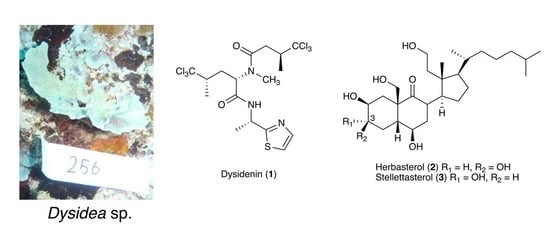
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Isolation of Compounds 1–3
3.3. Cell Culture
3.4. Assay for ALP in BMP-Treated C2C12(R206H) Cells
3.5. Cytotoxicity
3.6. Reporter Gene Assay for Monitoring BMP Signaling
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peltier, L.F.; Freke, J. A case of extraordinary exostoses on the back of a boy. 1740. John Freke (1688–1756). Clin. Orthop. Relat. Res. 1998, 346, 5–6. [Google Scholar] [CrossRef]
- Kaplan, F.S.; McCluskey, W.; Hahn, G.; Tabas, J.A.; Muenke, M.; Zasloff, M.A. Genetic transmission of fibrodysplasia ossificans progressiva. Report of a family. J. Bone Jt. Surg. Am. 1993, 75, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Glaser, D.L.; Economides, A.E.; Wang, L.; Liu, X.; Kimble, R.D.; Fandl, J.P.; Wilson, J.M.; Stahl, N.; Kaplan, F.S.; Shore, E.M. In vivo somatic cell gene transfer of an engineered Noggin mutein prevents BMP4-induced heterotopic ossification. J. Bone Jt. Surg. Am. 2003, 85, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Shore, E.M.; Xu, M.; Feldmn, G.J.; Fenstermacher, D.A.; Cho, T.J.; Choi, I.H.; Connor, J.M.; Delai, P.; Triffitt, J.T.; Urtizberea, J.A.; et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006, 38, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.B.; Deng, D.Y.; Lai, C.S.; Hong, C.C.; Cuny, G.D.; Bouxsein, M.L.; Hong, D.W.; McManus, P.M.; Katagiri, T.; Sachidanandan, C.; et al. BMP type I receptor inhibition reduces heterotopic ossification. Nat. Med. 2008, 14, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Tsukamoto, S.; Nakachi, Y.; Kuratani, M. Recent topics in fibrodysplasia ossificans progressiva. Endocrinol. Metab. 2018, 33, 331–338. [Google Scholar] [CrossRef]
- Fukuda, T.; Uchida, R.; Inoue, H.; Ohte, S.; Yamazaki, H.; Matsuda, D.; Katagiri, T.; Tomoda, H. Fungal pyrrolidine-containing metabolites inhibit alkaline phosphatase activity in bone morphogenetic protein-stimulated myoblastoma cells. Acta Pharm. Sin. B 2012, 2, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, T.; Uchida, R.; Ohte, S.; Inoue, H.; Yamazaki, H.; Matsuda, D.; Nonaka, K.; Masuma, R.; Katagiri, T.; Tomoda, H. Trichocyalides A and B, new inhibitors of alkaline phosphatase activity in bone morphogenetic protein-stimulated myoblasts, produced by Trichoderma sp. FKI-5513. J. Antibiot. 2012, 65, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Uchida, R.; Nakai, M.; Ohte, S.; Onaka, H.; Katagiri, T.; Tomoda, H. 5-Prenyltryptophol, a new inhibitor of bone morphogenetic protein-induced alkaline phosphatase expression in myoblasts, produced by Streptomyces colinus subsp. albescens HEK608. J. Antibiot. 2014, 67, 589–591. [Google Scholar] [CrossRef] [Green Version]
- Uchida, R.; Lee, D.; Suwa, I.; Ohtawa, M.; Watanabe, N.; Demachi, A.; Ohte, S.; Katagiri, T.; Nagamitsu, T.; Tomoda, H. Scopranones with Two Atypical Scooplike Moieties Produced by Streptomyces sp. BYK-11038. Org. Lett. 2017, 19, 5980–5983. [Google Scholar] [CrossRef]
- Ohte, S.; Shiokawa, T.; Koyama, N.; Katagiri, T.; Imada, C.; Tomoda, H. A new diketopiperazine-like inhibitor of bone morphogenetic protein-induced osteoblastic differentiation produced by marine-derived Aspergillus sp. BFM-0085. J. Antibiot. 2020, 73, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–48, and previous reports in this series. [Google Scholar] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223, and previous reports in this series. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, R.; Lidgard, R.O.; Wells, R.J.; Vetter, W. A novel hexachloro-metabolite from the sponge Dysidea herbacea. Tetrahedron Lett. 1977, 36, 3183–3186. [Google Scholar] [CrossRef]
- Lindenthal, S.; Lecat-Guillet, N.; Ondo-Mendez, A.; Ambroise, Y.; Rousseau, B.; Pourcher, T. Characterization of small-molecule inhibitors of the sodium iodide symporter. J. Endcrinol. 2009, 200, 357–365. [Google Scholar] [CrossRef]
- Deschamps, J.D.; Gautschi, J.T.; Whitman, S.; Johnson, T.A.; Gassner, N.C.; Crews, P.; Holman, T.R. Discovery of platelet-type 12-human lipoxygenase selective inhibitors by high-throughput screening of structurally diverse libraries. Bioorg. Med. Chem. 2007, 15, 6900–6908. [Google Scholar] [CrossRef] [Green Version]
- Capon, R.J.; Faulkner, D.J. Herbasterol, an ichthyotoxic 9, 11-secosterol from the sponge Dysidea herbacea. J. Org. Chem. 1985, 50, 4771–4773. [Google Scholar] [CrossRef]
- Li, H.; Matsunaga, S.; Fusetani, N. A new 9, 11-secosterol, stellettasterol from a marine sponge Stelletta sp. Experientia 1994, 50, 771–773. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, T.; Imada, M.; Yanai, T.; Suda, T.; Takahashi, N.; Kamiho, R. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells 2002, 7, 949–960. [Google Scholar] [CrossRef] [PubMed]


| Compound | IC50 (μM) | ||
|---|---|---|---|
| ALP a | Cytotoxicity | BMP b Signaling | |
| 1 | 2.3 | >18.4 | >18.4 |
| 2 | 4.3 | >21.4 | >21.4 |
| 3 | 4.2 | >21.4 | >21.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, H.; Ohte, S.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Abdjul, D.B.; Maarisit, W.; Kapojos, M.M.; Namikoshi, M.; Katagiri, T.; et al. Screening for Small Molecule Inhibitors of BMP-Induced Osteoblastic Differentiation from Indonesian Marine Invertebrates. Mar. Drugs 2020, 18, 606. https://0-doi-org.brum.beds.ac.uk/10.3390/md18120606
Yamazaki H, Ohte S, Rotinsulu H, Wewengkang DS, Sumilat DA, Abdjul DB, Maarisit W, Kapojos MM, Namikoshi M, Katagiri T, et al. Screening for Small Molecule Inhibitors of BMP-Induced Osteoblastic Differentiation from Indonesian Marine Invertebrates. Marine Drugs. 2020; 18(12):606. https://0-doi-org.brum.beds.ac.uk/10.3390/md18120606
Chicago/Turabian StyleYamazaki, Hiroyuki, Satoshi Ohte, Henki Rotinsulu, Defny S. Wewengkang, Deiske A. Sumilat, Delfly B. Abdjul, Wilmar Maarisit, Magie M. Kapojos, Michio Namikoshi, Takenobu Katagiri, and et al. 2020. "Screening for Small Molecule Inhibitors of BMP-Induced Osteoblastic Differentiation from Indonesian Marine Invertebrates" Marine Drugs 18, no. 12: 606. https://0-doi-org.brum.beds.ac.uk/10.3390/md18120606