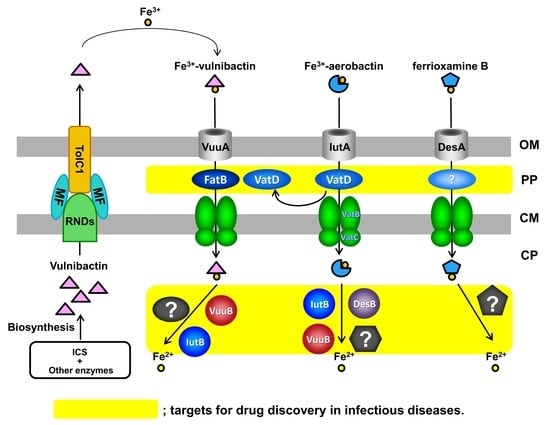
Iron-Utilization System in Vibrio vulnificus M2799
Abstract
:1. Introduction
2. Proteomic Analysis under Iron-Repleted and Low-Iron Conditions
3. Growth of the Deletion Mutants under Low-Iron Conditions
4. VatD in the Ferric-Vulnibactin Utilization System
5. IutB in the Ferric-Vulnibactin Utilization System
6. RND Proteins in the Vulnibactin-Export System
7. Heme-Acquisition System
8. New Targets for Antimicrobial Agents
9. Conclusion and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukhalfa, H.; Crumbliss, A.L. Chemical aspects of siderophore mediated iron transport. BioMetals 2002, 15, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C.; Dover, L.G. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 2000, 54, 881–941. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator—Siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef]
- Oliver, J.D. The Biology of Vibrio vulnificus. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Tacket, C.O.; Brenner, F.; Blake, P.A. Clinical features and an epidemiological study of Vibrio vulnificus infections. J. Infect. Dis. 1984, 149, 558–561. [Google Scholar] [CrossRef]
- Miyoshi, N.; Shimizu, C.; Miyoshi, S.I.; Shinoda, S. Purification and characterization of Vibrio vulnificus protease. Microbiol. Immunol. 1987, 31, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kothary, M.H.; Kreger, A.S. Purification and characterization of an elastolytic protease of Vibrio vulnifus. J. Gen. Microbiol. 1987, 133, 1783–1791. [Google Scholar]
- Simpson, L.M.; White, V.K.; Zane, S.F.; Oliver, J.D. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect. Immun. 1987, 55, 269–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwin, C.M.; Rayback, T.W.; Skinner, J. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect. Immun. 1996, 64, 2834–2838. [Google Scholar] [CrossRef] [Green Version]
- Litwin, C.M.; Byrne, B.L. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: Regulation of expression and determination of the gene sequence. Infect. Immun. 1998, 66, 3134–3141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, J.S.; Jeong, H.G.; Satchell, K.J.F. Vibrio vulnificus rtxA1 gene recombination generates toxin variants with altered potency during intestinal infection. Proc. Natl. Acad. Sci. USA 2011, 108, 1645–1650. [Google Scholar] [CrossRef] [Green Version]
- Choi, G.; Jang, K.K.; Lim, J.G.; Lee, Z.W.; Im, H.; Choi, S.H. The transcriptional regulator IscR integrates host-derived nitrosative stress and iron starvation in activation of the vvhBA operon in Vibrio vulnificus. J. Biol. Chem. 2020, 295, 5350–5361. [Google Scholar] [CrossRef]
- Oh, M.H.; Lee, S.M.; Lee, D.H.; Choi, S.H. Regulation of the Vibrio vulnificus hupA gene by temperature alteration and cyclic AMP receptor protein and evaluation of its role in virulence. Infect. Immun. 2009, 77, 1208–1215. [Google Scholar] [CrossRef] [Green Version]
- Roig, F.J.; González-Candelas, F.; Sanjuán, E.; Fouz, B.; Feil, E.J.; Llorens, C.; Baker-Austin, C.; Oliver, J.D.; Danin-Poleg, Y.; Gibas, C.J.; et al. Phylogeny of Vibrio vulnificus from the analysis of the core-genome: Implications for intra-species taxonomy. Front. Microbiol. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Mitsuo, E.; Hayashi, N.; Hikita, Y.; Nakao, H.; Yamamoto, S.; Miyamoto, K.; Tsujibo, H. Vibrio vulnificus damages macrophages during the early phase of infection. Infect. Immun. 2007, 75, 4592–4596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okujo, N.; Saito, M.; Yamamoto, S.; Yoshida, T.; Miyoshi, S.; Shinoda, S. Structure of vulnibactin, a new polyamine-containing siderophore from Vibrio vulnificus. BioMetals 1994, 7, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Verma, V.; Jeong, K.; Kim, S.Y.; Jung, C.H.; Lee, S.E.; Rhee, J.H. Molecular characterization of vulnibactin biosynthesis in Vibrio vulnificus indicates the existence of an alternative siderophore. Front. Microbiol. 2014, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.M.; Park, R.Y.; Park, J.H.; Sun, H.Y.; Bai, Y.H.; Ryu, P.Y.; Kim, S.Y.; Rhee, J.H.; Shin, S.H. Vibrio vulnificus vulnibactin, but not metalloprotease VvpE, is essentially required for iron-uptake from human holotransferrin. Biol. Pharm. Bull. 2006, 29, 911–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, A.C.D.; Litwin, C.M. Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect. Immun. 2000, 68, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Kawano, H.; Miyamoto, K.; Sakaguchi, I.; Myojin, T.; Moriwaki, M.; Tsuchiya, T.; Tanabe, T.; Yamamoto, S.; Tsujibo, H. Role of periplasmic binding proteins, FatB and VatD, in the vulnibactin utilization system of Vibrio vulnificus M2799. Microb. Pathog. 2013, 65, 73–81. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kosakai, K.; Ikebayashi, S.; Tsuchiya, T.; Yamamoto, S.; Tsujibo, H. Proteomic analysis of Vibrio vulnificus M2799 grown under iron-repleted and iron-depleted conditions. Microb. Pathog. 2009, 46, 171–177. [Google Scholar] [CrossRef]
- Tanabe, T.; Naka, A.; Aso, H.; Nakao, H.; Narimatsu, S.; Inoue, Y.; Ono, T.; Yamamoto, S. A novel aerobactin utilization cluster in Vibrio vulnificus with a gene involved in the transcription regulation of the iutA homologue. Microbiol. Immunol. 2005, 49, 823–834. [Google Scholar] [CrossRef]
- Tanabe, T.; Takata, N.; Naka, A.; Moon, Y.H.; Nakao, H.; Inoue, Y.; Narimatsu, S.; Yamamoto, S. Identification of an AraC-like regulator gene required for induction of the 78-kDa ferrioxamine B receptor in Vibrio vulnificus. FEMS Microbiol. Lett. 2005, 249, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Hantke, K. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 2001, 4, 172–177. [Google Scholar] [CrossRef]
- Pajuelo-Gamez, D.; Hernández-Cabanyero, C.; Sanjuan, E.; Lee, C.-T.; Silva-Hernández, F.X.; Hor, L.-I.; MacKenzie, S.; Amaro, C. Iron and Fur in the life cycle of the zoonotic pathogen Vibrio vulnificus. Environ. Microbiol. 2016, 18, 4005–4022. [Google Scholar] [CrossRef]
- Keen, N.T.; Tamaki, S.; Kobayashi, D.; Trollinger, D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 1988, 70, 191–197. [Google Scholar] [CrossRef]
- Miyano, N.; Igarashi, T.; Kawano, H.; Miyamoto, K.; Tsuchiya, T.; Tomoo, K.; Tsujibo, H. Expression, purification, crystallization and X-ray crystallographic analysis of the periplasmic binding protein VatD from Vibrio vulnificus M2799. Acta Crystallogr. Sect. Struct. Biol. Commun. 2015, 71, 1078–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, H.; Miyamoto, K.; Negoro, M.; Zushi, E.; Tsuchiya, T.; Tanabe, T.; Funahashi, T.; Tsujibo, H. IutB participates in the ferric-vulnibactin utilization system in Vibrio vulnificus M2799. BioMetals 2017, 30, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Miyamoto, K.; Nagaoka, K.; Tsujibo, H.; Funahashi, T. Binding of AraC-type activator DesR to the promoter region of Vibrio vulnificus ferrioxamine B receptor gene. Biol. Pharm. Bull. 2021, 44, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Okai, N.; Miyamoto, K.; Tomoo, K.; Tsuchiya, T.; Komano, J.; Tanabe, T.; Funahashi, T.; Tsujibo, H. VuuB and IutB reduce ferric-vulnibactin in Vibrio vulnificus M2799. BioMetals 2020, 33, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Bleuel, C.; Große, C.; Taudte, N.; Scherer, J.; Wesenberg, D.; Krauß, G.J.; Nies, D.H.; Grass, G. TolC is involved in enterobactin efflux across the outer membrane of Escherichia coli. J. Bacteriol. 2005, 187, 6701–6707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, H.; Miyamoto, K.; Yasunobe, M.; Murata, M.; Myojin, T.; Tsuchiya, T.; Tanabe, T.; Funahashi, T.; Sato, T.; Azuma, T.; et al. The RND protein is involved in the vulnibactin export system in Vibrio vulnificus M2799. Microb. Pathog. 2014, 75, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Nakashima, R.; Sakurai, K. Structural basis of RND-type multidrug exporters. Front. Microbiol. 2015, 6, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Guo, R.H.; Rhee, J.H.; Kim, Y.R. TolCV1 has multifaceted roles during Vibrio vulnificus infection. Front. Cell. Infect. Microbiol. 2021, 11, 673222. [Google Scholar] [CrossRef]
- Tong, Y.; Guo, M. Bacterial heme-transport proteins and their heme-coordination modes. Arch. Biochem. Biophys. 2009, 481, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, H.; Miyamoto, K.; Yasunobe, M.; Murata, M.; Yamahata, E.; Yamaguchi, R.; Miyaki, Y.; Tsuchiya, T.; Tanabe, T.; Funahashi, T.; et al. Identification of the heme acquisition system in Vibrio vulnificus M2799. Microb. Pathog. 2018, 117, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Alice, A.F.; Naka, H.; Crosa, J.H. Global gene expression as a function of the iron status of the bacterial cell: Influence of differentially expressed genes in the virulence of the human pathogen Vibrio vulnificus. Infect. Immun. 2008, 76, 4019–4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, S.M.; Mey, A.R.; Wyckoff, E.E. Vibrio Iron Transport: Evolutionary adaptation to life in multiple environments. Microbiol. Mol. Biol. Rev. 2016, 80, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyckoff, E.E.; Mey, A.R.; Leimbach, A.; Fisher, C.F.; Payne, S.M. Characterization of ferric and ferrous iron transport systems in Vibrio cholerae. J. Bacteriol. 2006, 188, 6515–6523. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, K.; Kawano, H.; Okai, N.; Hiromoto, T.; Miyano, N.; Tomoo, K.; Tsuchiya, T.; Komano, J.; Tanabe, T.; Funahashi, T.; et al. Iron-Utilization System in Vibrio vulnificus M2799. Mar. Drugs 2021, 19, 710. https://0-doi-org.brum.beds.ac.uk/10.3390/md19120710
Miyamoto K, Kawano H, Okai N, Hiromoto T, Miyano N, Tomoo K, Tsuchiya T, Komano J, Tanabe T, Funahashi T, et al. Iron-Utilization System in Vibrio vulnificus M2799. Marine Drugs. 2021; 19(12):710. https://0-doi-org.brum.beds.ac.uk/10.3390/md19120710
Chicago/Turabian StyleMiyamoto, Katsushiro, Hiroaki Kawano, Naoko Okai, Takeshi Hiromoto, Nao Miyano, Koji Tomoo, Takahiro Tsuchiya, Jun Komano, Tomotaka Tanabe, Tatsuya Funahashi, and et al. 2021. "Iron-Utilization System in Vibrio vulnificus M2799" Marine Drugs 19, no. 12: 710. https://0-doi-org.brum.beds.ac.uk/10.3390/md19120710