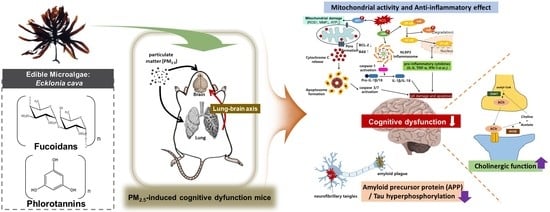
Ecklonia cava Attenuates PM2.5-Induced Cognitive Decline through Mitochondrial Activation and Anti-Inflammatory Effect
Abstract
:1. Introduction
2. Results
2.1. Behavioral Test
2.2. Anti-Inflammatory Effect
2.3. Inhibitory Effect of Lipid Peroxidation
2.4. Mitochondrial Function
2.4.1. Mitochondrial Function in Lung Tissue
2.4.2. Mitochondrial Function in Brain Tissue
2.5. Cognitive Function-Mediated Molecule Analysis
2.5.1. Aβ Production/tau Hyperphosphorylation Signaling Pathways
2.5.2. Cholinergic Function
2.6. Main Compound Analysis
2.6.1. Chemical Composition of Polysaccharide
2.6.2. Phenolic Compounds Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. In Vivo Experimental Design
4.3.1. Animals
4.3.2. Y-Maze Test
4.3.3. Passive Avoidance Test
4.3.4. Morris Water Maze Test
4.4. Biochemicals Analysis
4.4.1. Preparation of the Tissue
4.4.2. Western Blot Assay
4.4.3. Cytokine Contents
4.4.4. MDA Content
4.5. Mitochondrial Activity
4.5.1. Isolation of Mitochondria
4.5.2. Mitochondrial ROS Content
4.5.3. Measurement of MMP
4.5.4. ATP Level
4.6. Measurement of Cholinergic Function
4.6.1. AChE Activity
4.6.2. ACh Contents
4.7. Major Components Analysis
4.7.1. Determination of Total Polysaccharide Contents
4.7.2. Determination of Average Molecular Weight
4.7.3. Determination of Sulfate
4.7.4. Monosaccharide Composition
4.7.5. Major Phenolic Compound Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.P. Research and policy directions against ambient fine particles. J. Korean Soc. Atmos. Environ. 2017, 33, 191–204. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar]
- Zhu, Z.; Wu, H.; Su, W.; Shi, R.; Li, P.; Liao, Y.; Wang, Y.; Li, P. Effects of total flavonoids from Exocarpium citri Grandis on air pollution particle-induced pulmonary inflammation and oxidative stress in mice. J. Food Sci. 2019, 84, 3843–3849. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nakagawa, S. Planetary health and the future of human capacity: The increasing impact of planetary distress on the human brain. Challenges 2018, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Mumaw, C.L.; Levesque, S.; McGraw, C.; Robertson, S.; Lucas, S.; Stafflinger, J.E.; Campen, M.J.; Hall, P.; Norenberg, J.P.; Anderson, T.; et al. Microglial priming through the lung—brain axis: The role of air pollution-induced circulating factors. FASEB J. 2016, 30, 1880–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. 2020, 27, 42390–42404. [Google Scholar] [CrossRef]
- Block, M.L.; Wu, X.; Pei, Z.; Li, G.; Wang, T.; Qin, L.; Wilson, B.; Yang, J.; Hong, J.S.; Veronesi, B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: The role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2008, 18, 1618–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón-Garcidueñas, L.; Kavanaugh, M.; Block, M.; D’Angiulli, A.; Delgado-Chávez, R.; Torres-Jardón, R.; González-Maciel, A.; Reynoso-Robles, R.; Osnaya, N.; Villarreal-Calderon, R.; et al. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J. Alzheimers Dis. 2012, 28, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Chew, S.; Kolosowska, N.; Saveleva, L.; Malm, T.; Kanninen, K.M. Impairment of mitochondrial function by particulate matter: Implications for the brain. Neurochem. Int. 2020. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Zhou, Y.; Li, Y.; Qin, Y.; Xu, Y. Neurodevelopmental toxicity induced by maternal PM2.5 exposure and protective effects of quercetin and vitamin C. Chemosphere 2018, 213, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.N.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Okolie, C.L.; CK Rajendran, S.R.; Udenigwe, C.C.; Aryee, A.N.; Mason, B. Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J. Food Biochem. 2017, 41. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Potential beneficial actions of fucoidan in brain and liver injury, disease, and intoxication—Potential implication of sirtuins. Mar. Drugs 2020, 18, 242. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, Á.; Andrade, P.B.; Valentão, P.; Pereiraa, D.M.; Ferreres, F. Profiling phlorotannins from Fucus spp. of the Northern Portuguese coastline: Chemical approach by HPLC-DAD-ESI/MSn and UPLC-ESI-QTOF/MS. Algal Res. 2018, 29, 113–120. [Google Scholar] [CrossRef]
- Cho, H.M.; Doan, T.P.; Ha, T.K.Q.; Kim, H.W.; Lee, B.W.; Pham, H.T.T.; Cho, T.O.; Oh, W.K. Dereplication by high-performance liquid chromatography (HPLC) with quadrupole-time-of-flight mass spectroscopy (qTOF-MS) and antiviral activities of phlorotannins from Ecklonia cava. Mar. Drugs 2019, 17, 149. [Google Scholar] [CrossRef] [Green Version]
- Riva, D.; Magalhaes, C.B.; Lopes, A.; Lancas, T.; Mauad, T.; Malm, O.; Valenca, S.S.; Saldiva, P.H.; Faffe, D.S.; Zin, W.A. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal. Toxicol. 2011, 23, 257–267. [Google Scholar] [CrossRef]
- Heusinkveld, H.J.; Wahle, T.; Campbell, A.; Westerink, R.H.; Tran, L.; Johnston, H.; Stone, V.; Cassee, F.R.; Schins, R.P. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 2016, 56, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Q.; Ma, J.; Zhao, Y. PM2.5 impairs neurobehavior by oxidative stress and myelin sheaths injury of brain in the rat. Environ. Pollut. 2018, 242, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Ee, N.; Peters, J.; Booth, A.; Mudway, I.; Anstey, K.J. Air pollution and dementia: A systematic review. J. Alzheimers Dis. 2019, 70, S145–S163. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Springer: New York, NY, USA, 2014; Volume 817, pp. 195–219. [Google Scholar]
- Becker, S.; Dailey, L.; Soukup, J.M.; Silbajoris, R.; Devlin, R.B. TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol. Appl. Pharmacol. 2005, 203, 45–52. [Google Scholar] [CrossRef]
- Bauer, R.N.; Diaz-Sanchez, D.; Jaspers, I. Effects of air pollutants on innate immunity: The role of Toll-like receptors and nucleotide-binding oligomerization domain–like receptors. J. Allergy Clin. Immunol. 2012, 129, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.J.; Ryter, S.W. Inflammasomes: Molecular regulation and implications for metabolic and cognitive diseases. Mol. Cells 2014, 37, 441–448. [Google Scholar] [CrossRef]
- Sahu, B.; Sandhir, R.; Naura, A.S. Two hit induced acute lung injury impairs cognitive function in mice: A potential model to study cross talk between lung and brain. Brain Behav. Immun. 2018, 73, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, H.S.; Kim, S.Y.; Fernando, I.S.; Wang, L.; Abetunga, D.T.U.; Kim, W.S.; Lee, D.S.; Jeon, Y.J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr. Polym. 2019, 224. [Google Scholar] [CrossRef]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J. Why Is the Nervous System Vulnerable to Oxidative Stress? In Oxidative Stress and Free Radical Damage in Neurology; Oxidative Stress in Applied Basic Research and Clinical Practice; Gadoth, N., Göbel, H., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 25, pp. 19–27. [Google Scholar]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Esposito, E.; Petrosino, S.; Cuzzocrea, S. Anti-inflammatory and neuroprotective effects of Co-UltraPEALut in a mouse model of vascular dementia. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Antioch, I.; Dobrin, R.; Timofte, D. Oxidative stress implications in the affective disorders: Main biomarkers, animal models relevance, genetic perspectives, and antioxidant approaches. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Kou, X.; Geng, H.; Xie, J.; Yang, Z.; Zhang, Y.; Cai, Z.; Dong, C. Effect of ambient PM2.5 on lung mitochondrial damage and fusion/fission gene expression in rats. Chem. Res. Toxicol. 2015, 28, 408–418. [Google Scholar] [CrossRef]
- Cho, D.H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-nitrosylation of Drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Concannon, C.G.; Tuffy, L.P.; Weisová, P.; Bonner, H.P.; Dávila, D.; Bonner, C.; Devocelle, M.C.; Strasser, A.; Ward, M.W.; Prehn, J.H. AMP kinase–mediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J. Cell Biol. 2010, 189, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Youn, K.; Kim, D.H.; Ahn, M.R.; Yoon, E.; Kim, O.Y.; Jun, M. Anti-neuroinflammatory property of phlorotannins from Ecklonia cava on Aβ25-35-induced damage in PC12 cells. Mar. Drugs 2019, 17, 7. [Google Scholar] [CrossRef] [Green Version]
- Bedse, G.; Di Domenico, F.; Serviddio, G.; Cassano, T. Aberrant insulin signaling in Alzheimer’s disease: Current knowledge. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.H.; Choi, J.S.; Nam, T.J. Fucosterol from an edible brown alga Ecklonia stolonifera prevents soluble amyloid beta-induced cognitive dysfunction in aging rats. Mar. Drugs 2018, 16, 368. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.; Hartley, D.M.; Lashuel, H.A. Preparation and characterization of toxic Aβ aggregates for structural and functional studies in Alzheimer’s disease research. Nat. Protoc. 2010, 5, 1186–1209. [Google Scholar] [CrossRef]
- Terry, A.V.; Buccafusco, J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Lee, J.; Jun, M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava—An In Vitro and in Silico Study. Mar. Drugs 2019, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Martorana, A.; Di Lorenzo, F.; Manenti, G.; Semprini, R.; Koch, G. Homotaurine induces measurable changes of short latency afferent inhibition in a group of mild cognitive impairment individuals. Front. Aging Neurosci. 2014, 6. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2018, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Food. 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.S.; Jeon, Y.J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef]
- Manikandan, R.; Parimalanandhini, D.; Mahalakshmi, K.; Beulaja, M.; Arumugam, M.; Janarthanan, S.; Palanisamy, S.; You, S.G.; Prabhu, N.M. Studies on isolation, characterization of fucoidan from brown algae Turbinaria decurrens and evaluation of it’s in vivo and in vitro anti-inflammatory activities. Int. J. Biol. Macromol. 2020, 160, 1263–1276. [Google Scholar] [CrossRef]
- Liu, X.; Cao, S.; Zhang, X. Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Kim, E.A.; Son, K.T.; Jeon, Y.J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef]
- Han, M.; Hur, Y.; Hwang, J.; Park, J. Biological effects of blood–brain barrier disruption using a focused ultrasound. Biomed. Eng. Lett. 2017, 7, 115–120. [Google Scholar] [CrossRef]
- Yang, E.J.; Mahmood, U.; Kim, H.; Choi, M.; Choi, Y.; Lee, J.P.; Cho, J.Y.; Hyun, J.W.; Kim, Y.S.; Chang, M.J.; et al. Phloroglucinol ameliorates cognitive impairments by reducing the amyloid β peptide burden and pro-inflammatory cytokines in the hippocampus of 5XFAD mice. Free Radic. Biol. Med. 2018, 126, 221–234. [Google Scholar] [CrossRef]
- Kang, I.J.; Jang, B.G.; In, S.; Choi, B.; Kim, M.; Kim, M.J. Phlorotannin-rich Ecklonia cava reduces the production of beta-amyloid by modulating alpha-and gamma-secretase expression and activity. Neurotoxicology 2013, 34, 16–24. [Google Scholar] [CrossRef]
- Yang, E.J.; Ahn, S.; Ryu, J.; Choi, M.S.; Choi, S.; Chong, Y.H.; Hyun, J.W.; Chang, M.J.; Kim, H.S. Phloroglucinol attenuates the cognitive deficits of the 5XFAD mouse model of Alzheimer’s disease. PLoS ONE 2015, 10, e0135686. [Google Scholar] [CrossRef]
- Um, M.Y.; Lim, D.W.; Son, H.J.; Cho, S.; Lee, C. Phlorotannin-rich fraction from Ishige foliacea brown seaweed prevents the scopolamine-induced memory impairment via regulation of ERK-CREB-BDNF pathway. J. Funct. Food. 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 2 May 2018).
- List of Most-Polluted Cities by Particulate Matter Concentration. Available online: https://en.wikipedia.org/wiki/List_of_most-polluted_cities_by_particulate_matter_concentration (accessed on 2 September 2020).
- Morris, R. Developments of a water-maze procedure for studying a spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, D.M.; Li, S.Q.; Wu, W.L.; Zhu, X.Y.; Wang, Y.; Yuan, H.Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem. Res. 2014, 39, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- Vincent, D.; Segonzac, G.; Vincent, M.C. Colorimetric determination of acetylcholine by the hestrin hydroxylamine reaction and its application in pharmacy. Ann. Pharm. Fr. 1958, 16, 179–185. [Google Scholar]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.O.; Kim, G.H.; Heo, H.J. Fucoidan-rich substances from Ecklonia cava improve trimethyltin-induced cognitive dysfunction via down-regulation of amyloid β production/tau hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef] [Green Version]







| Total Polysaccharide (%) | Average Molecular Weight (kDa) | Sulfate (%) | Relative Area (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Fucose | Rhamnose | Galactose | Glucose | Xylose | Others | |||
| 34.26 | 160.13 | 17.03 | 9.76 | 16.03 | 6.53 | 6.65 | 48.97 | 12.06 |
| No. | RT (min) | m/z [M − H]− | LC-MS/MS Fragments | Proposed Compounds |
|---|---|---|---|---|
| 1 | 2.60 | 497.11278 | 327, 265, 231, 139 | isometric tetramer (phlorotannin oligomer) |
| 2 | 2.93 | 497.11278 | 371, 353, 231, 229, 138, 125 | isometric tetramer (phlorotannin oligomer) |
| 3 | 3.21 | 371.04088 | 353, 263, 245, 201 | Eckol |
| 4 | 3.25 | 495.09875 | 477, 387, 263, 244,231,229,201 | 2-Phloroeckol |
| 5 | 3.34 | 741.13508 | 723, 490, 477, 244, 229, 201 | 6′6′-Bieckol |
| 6 | 3.62 | 741.13508 | 615, 493, 491, 477, 369, 261, 229, 201 | Dieckol |
| 7 | 3.79 | 601.11104 | 493, 492, 385, 366, 244, 299 | Phlorofuroeckol A |
| 8 | 4.13 | 973.19218 | 741, 602, 601, 493, 370, 229 | 2,7″-Phloroglucinol 6,6′-bieckol (PHB) |
| 9 | 4.25 | 973.19218 | 829, 707 493, 479, 353, 335, 229 | 974-A |
| 10 | 6.91 | 642.4498 | 363, 362, 279, 99, 85 | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, H.-J.; Heo, H.J. Ecklonia cava Attenuates PM2.5-Induced Cognitive Decline through Mitochondrial Activation and Anti-Inflammatory Effect. Mar. Drugs 2021, 19, 131. https://0-doi-org.brum.beds.ac.uk/10.3390/md19030131
Park SK, Kang JY, Kim JM, Kim H-J, Heo HJ. Ecklonia cava Attenuates PM2.5-Induced Cognitive Decline through Mitochondrial Activation and Anti-Inflammatory Effect. Marine Drugs. 2021; 19(3):131. https://0-doi-org.brum.beds.ac.uk/10.3390/md19030131
Chicago/Turabian StylePark, Seon Kyeong, Jin Yong Kang, Jong Min Kim, Hyun-Jin Kim, and Ho Jin Heo. 2021. "Ecklonia cava Attenuates PM2.5-Induced Cognitive Decline through Mitochondrial Activation and Anti-Inflammatory Effect" Marine Drugs 19, no. 3: 131. https://0-doi-org.brum.beds.ac.uk/10.3390/md19030131