Screening of In-Vitro Anti-Inflammatory and Antioxidant Activity of Sargassum ilicifolium Crude Lipid Extracts from Different Coastal Areas in Indonesia
Abstract
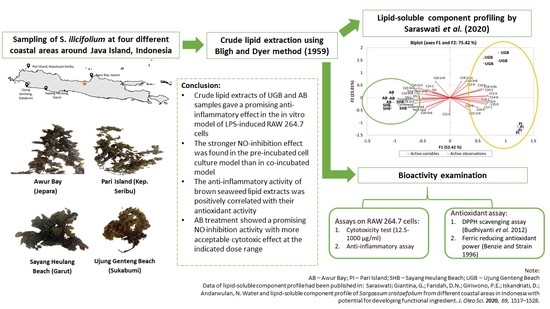
:1. Introduction
2. Results
2.1. Cell Viability after Treatment of S. ilicifolium Crude Lipid Extract
2.2. Anti-Inflammatory Activity of S. ilicifolium Crude Lipid Extract
2.3. DPPH Radical Scavenging and Ferric Reducing Ability of S. ilicifolium Crude Lipid Extract
3. Materials and Methods
3.1. Materials
3.2. Brown Seaweed Sampling and Sample Preparation
3.3. Preparation of Crude Lipid Extract and The Chemical Characterization
3.4. Bioactivity Examination
3.4.1. Cell Culture and Cell Viability Assay
3.4.2. Anti-Inflammatory Activity Assay
3.4.3. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Scavenging Assay
3.4.4. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Marine and Fisheries, Republic of Indonesia. Marine and Fisheries in Figures 2018; The Center for Data, Statistics, and Information of Ministry of Marine and Fisheries: Jakarta, Indonesia, 2018.
- Ferdouse, F.; Holdt, L.S.; Smith, R.; Murua, P.; Yang, Z. The Global Status of Seaweed Production, Trade and Utilization; Food and Agriculture Organization of The United Nations: Rome, Italy, 2018. [Google Scholar]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; Mcsorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Potential targets for anti-inflammatory and anti-allergic activities of marine algae: An overview. Inflamm. Allergy Drug Targets 2012, 11, 90–101. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in traditional chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum seaweed as a source of anti-inflammatory substances and the potential insight of the tropical species: A review. Mar. Drugs 2019, 17, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadeniz, F.; Lee, S.G.; Oh, J.H.; Kim, J.A.; Kong, C.S. Inhibition of MMP-2 and MMP-9 activities by solvent-partitioned Sargassum horneri extracts. Fish. Aquat. Sci. 2018, 21, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gwon, W.G.; Lee, S.G.; Kim, J.I.; Kim, Y.M.; Kim, S.B.; Kim, H.R. Hexane fraction from the ethanolic extract of Sargassum serratifolium suppresses cell adhesion molecules via regulation of NF-κB and Nrf2 pathway in human umbilical vein endothelial cells. Fish. Aquat. Sci. 2019, 22, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jayawardena, T.U.; Kim, H.S.; Sanjeewa, K.K.A.; Kim, S.Y.; Rho, J.R.; Jee, Y.; Ahn, G.; Jeon, Y.J. Sargassum horneri and isolated 6-hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one (HTT); LPS-induced inflammation attenuation via suppressing NF-κB, MAPK and oxidative stress through Nrf2/HO-1 pathways in RAW 264.7 macrophages. Algal Res. 2019, 40, 101513. [Google Scholar] [CrossRef]
- Mun, O.J.; Kwon, M.S.; Karadeniz, F.; Kim, M.; Lee, S.H.; Kim, Y.Y.; Seo, Y.; Jang, M.S.; Nam, K.H.; Kong, C.S. Fermentation of Sargassum thunbergii by kimchi-derived Lactobacillus sp. SH-1 attenuates LPS-stimulated inflammatory response via downregulation of JNK. J. Food Biochem. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed biorefinery. Rev. Environ. Sci. Biotechnol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. Sargassum Ilicifolium (Turner) C. Agardh 1820. 2021. Available online: https://www.algaebase.org/search/species/detail/?species_id=4580 (accessed on 16 April 2020).
- Coppejans, E.; De Clerck, O.; Leliaert, F. Marine brown algae (Phaeophyta) from the north coast of Papua New Guinea, with a description of Dictyota magneana sp. nov. Cryptogam. Algol. 2001, 22, 15–40. [Google Scholar] [CrossRef]
- Soe-Htun, U.; Yoshida, T. Studies on morphological variations in Sargassum cristaefolium C. Agardh (Phaeophyta, Fucales). Jpn. J. Phycol. 1986, 34, 275–281. [Google Scholar]
- Wu, G.J.; Shiu, S.M.; Hsieh, M.C.; Tsai, G.J. Anti-inflammatory activity of a sulfated polysaccharide from the brown alga Sargassum cristaefolium. Food Hydrocoll. 2016, 53, 16–23. [Google Scholar]
- Simpi, C.; Nagathan, C.; Karajgi, S.; Kalyane, N. Evaluation of marine brown algae Sargassum ilicifolium extract for analgesic and anti-inflammatory activity. Pharmacogn. Res. 2013, 5, 146. [Google Scholar]
- Lavanya, R.; Seethalakshmi, S.; Gopal, V.; Chamundeeswari, D. Effect of crude sulphated polysaccharide from marine brown algae in TPA induced inflammation on poly morphonuclear leukocytes. Int. J. Pharm. Pharm. Sci. 2015, 7, 100–102. [Google Scholar]
- Jaswir, I.; Monsur, H.A.; Simsek, S.; Amid, A.; Alam, Z.; bin Salleh, M.N.; Tawakalit, A.-H.; Octavianti, F. Cytotoxicity and inhibition of nitric oxide in lipopolysaccharide-induced mammalian cell lines by aqueous extracts of brown seaweed. J. Oleo Sci. 2014, 63, 787–794. [Google Scholar] [PubMed] [Green Version]
- Monsur, A.H.; Jaswir, I.; Simsek, S.; Amid, A.; Alam, Z.; Tawakalit, A.-H. Cytotoxicity and inhibition of nitric oxide syntheses in LPS induced macrophage by water soluble fractions of brown seaweed. Food Hydrocoll. 2014, 42, 269–274. [Google Scholar]
- Rosdiana, A.; Rahmah, N.L. Potency of brown seaweed (Sargassum duplicatum Bory) ethanol and ethyl acetate fraction to malondialdehyde concentration decreasing and histological retriveal of IBD (inflammatory bowel disease) rat small intestinal jejunum. Media Vet. Med. 2011, 4, 57–64. [Google Scholar]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Oh, C.; Choi, Y.-U.; Yoon, K.-T.; Kang, D.-H.; Qian, Z.-J.; Choi, I.-W.; Jung, W.-K. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem. Toxicol. 2012, 50, 3336–3342. [Google Scholar]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. The anti-inflammatory properties of terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018, 3, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-rich extracts obtained from winemakingwaste streams as natural ingredients with cosmeceutical potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gany, S.A.; Tan, S.C.; Gan, S.Y. Anti-neuroinflammatory properties of Malaysian brown and green seaweeds. Int. J. Ind. Manuf. Eng. 2014, 8, 1269–1275. [Google Scholar]
- Hidalgo, M.; Martin-Santamaria, S.; Recio, I.; Sanchez-Moreno, C.; De Pascual-Teresa, B.; Rimbach, G.; De Pascual-Teresa, S. Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012, 7, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Chohan, M.; Naughton, D.P.; Jones, L.; Opara, E.I. An investigation of the relationship between the anti-inflammatory activity, polyphenolic content, and antioxidant activities of cooked and in vitro digested culinary herbs. Oxid. Med. Cell. Longev. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.W.F.; Ho, C.W.; Yong, W.T.L.; Abas, F.; Tan, T.B.; Tan, C.P. Extraction of phenolic antioxidants from four selected seaweeds obtained from Sabah. Int. Food Res. J. 2016, 23, 2363–2369. [Google Scholar]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar Drugs. 2020, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Budhiyanti, S.A.; Raharjo, S.; Marseno, D.W.; Lelana, I.Y.B. Antioxidant activity of brown algae Sargassum species extract from the coastline of Java island. Am. J. Agric. Biol. Sci. 2012, 7, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Rizzo, A.M. Characterization of antioxidant potential of seaweed extracts for enrichment of convenience food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and neuroprotective potential of the brown seaweed Bifurcaria bifurcata in an in vitro Parkinson’s disease model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective role of ternatin anthocyanins and quercetin glycosides from butterfly pea (Clitoria ternatea Leguminosae) blue flower petals against lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Fe3+–Fe2+ transformation method: An important antioxidant assay. In Advanced Protocols in Oxidative Stress III, Methods in Molecular Biology, vol 1208; Armstrong, D., Ed.; Springer Science+Business Media: New York, NY, USA, 2015; pp. 233–246. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G. In-vitro antioxidant properties of lipophilic antioxidant compounds from 3 brown seaweed. Antioxidants 2019, 8, 596. [Google Scholar] [CrossRef] [Green Version]
- Syad, A.N.; Shunmugiah, K.P.; Kasi, P.D. Antioxidant and anti-cholinesterase activity of Sargassum wightii. Pharm. Biol. 2013, 51, 1401–1410. [Google Scholar] [CrossRef]
- Adebayo, S.A.; Steel, H.C.; Shai, L.J.; Eloff, J.N. Investigation of the mechanism of anti-inflammatory action and cytotoxicity of a semipurified fraction and isolated compounds from the leaf of Peltophorum africanum (Fabaceae). J. Evid. Based Complement. Altern. Med. 2017, 22, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Somchit, N.; Kimseng, R.; Dhar, R.; Hiransai, P.; Changtam, C.; Suksamrarn, A.; Chunglok, W. Curcumin pyrazole blocks lipopolysaccharide-induced inflammation via suppression of JNK activation in RAW 264.7 macrophages. Asian Pac. J. Allergy Immunol. 2018, 36, 184–190. [Google Scholar]
- Muller, P.Y.; Milton, M.N. The determination and interpretation of the therapeutic index in drug development. Nat. Rev. Drug Discov. 2012, 11, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Osman, M.E.H. Seasonal fluctuation of photosynthetic pigments of most common red seaweeds species collected from Abu Qir, Alexandria, Egypt. Rev. Biol. Mar. Oceanogr. 2016, 51, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Gosch, B.J.; Paul, N.A.; de Nys, R.; Magnusson, M. Spatial, seasonal, and within-plant variation in total fatty acid content and composition in the brown seaweeds Dictyota bartayresii and Dictyopteris australis (Dictyotales, Phaeophyceae). J. Appl. Phycol. 2015, 27, 1607–1622. [Google Scholar] [CrossRef]
- Saraswati; Giantina, G.; Faridah, D.N.; Giriwono, P.E.; Iskandriati, D.; Andarwulan, N. Water and lipid-soluble component profile of Sargassum cristaefolium from different coastal areas in Indonesia with potential for developing functional ingredient. J. Oleo Sci. 2020, 69, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Foseid, L.; Devle, H.; Stenstrøm, Y.; Naess-andresen, C.F.; Ekeberg, D. Fatty acid profiles of stipe and blade from the Norwegian brown macroalgae Laminaria hyperborea with special reference to acyl glycerides, polar lipids, and free fatty acids. J. Lipids 2017, 2017, 102970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivikko, R. Brown Algal Phlorotannins: Improving and Applying Chemical Methods; University of Turku: Turku, Finland, 2008. [Google Scholar]
- Tasende, M.G. Fatty acid and sterol composition of gametophytes and sporophytes of Chondrus crispus (Gigartinaceae, Rhodophyta). Sci. Mar. 2000, 64, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. In-vitro anti-inflammatory activity, free radical (DPPH) scavenging, and ferric reducing ability (FRAP) of Sargassum cristaefolium lipid-soluble fraction and putative identification of bioactive compounds using UHPLC-ESI-ORBITRAP-MS/MS. Food Res. Int. 2020, 137, 109702. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between fatty acid profile and anti-inflammatory activity in common Australian seafood by-products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Susanto, E.; Fahmi, A.S.; Hosokawa, M.; Abe, M.; Miyashita, K. Lipids, fatty acids, and fucoxanthin content from temperate and tropical brown seaweeds. Aquat. Procedia 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Kamal, A.F.; Iskandriati, D.; Dilogo, I.H.; Siregar, N.C.; Hutagalung, E.U.; Susworo, R.; Yusuf, A.A.; Bachtiar, A. Biocompatibility of various hydroxyapatite scaffolds evaluated by proliferation of rat’s bone marrow mesenchymal stem cells: An in vitro study. Med. J. Indones. 2013, 22, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.-S.; Xiang, X.-W.; Jin, H.-X.; Guo, X.-Y.; Liu, L.-J.; Huang, Y.-N.; OuYang, X.-K.; Qu, Y.-L. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef]
- Rao, U.M.; Ahmad, B.A.; Mohd, K.S. In vitro nitric oxide scavenging and anti inflammatory activities of different solvent extract of various parts of Musa paradisiaca. Malaysian J. Anal. Sci. 2016, 20, 1191–1202. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Observed Parameters | Sample Origin | |||
|---|---|---|---|---|
| SHB | UGB | PI | AB | |
| Total lipid (g/100 g seaweed db) 1 | 3.55 ± 0.29 b 2 | 2.76 ± 0.28 a | 2.73 ± 0.19 a | 4.32 ± 0.09 c |
| Antioxidant activity (µmol TE/g seaweed db) 1 | 1.27 ± 0.01 c | 1.01 ± 0.00 b | 0.96 ± 0.03 a | 1.58 ± 0.001 d |
| Antioxidant activity (µmol TE/g lipid extract) 1 | 34.61 ± 0.17 a | 37.87 ± 0.08 b | 37.24 ± 1.15 b | 36.93 ± 0.18 b |
| DPPH scavenging effect (%) with extract concentration of 500 ppm 1 | 39.6 ± 0.15 a | 42.57 ± 0.08 b | 42.00 ± 1.05 b | 41.71 ± 0.17 b |
| FRAP (µmol FeSO4/g seaweed db) 1 | 23.44 ± 0.41 d | 25.98 ± 1.28 b | 16.32 ± 0.04 c | 29.24 ± 0.32 a |
| FRAP (µmol FeSO4/g lipid extract) 1 | 637.06 ± 11.12 a | 969.31 ± 47.88 c | 634.88 ± 1.50 a | 681.58 ± 7.48 b |
| Treatment | Pearson’s Correlation Coefficient |
|---|---|
| DPPH vs. FRAP | 0.484 |
| DPPH vs. NO Inhibition in Pre-incubated Model | 0.758 ** |
| FRAP vs. NO Inhibition in Pre-incubated Model | 0.749 ** |
| DPPH vs. NO Inhibition in Co-incubated Model | 0.794 ** |
| FRAP vs. NO Inhibition in Co-incubated Model | 0.819 ** |
| NO Inhibition in Pre-incubated Model vs NO Inhibition in Co-incubated Model | 0.865 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saraswati; Giriwono, P.E.; Iskandriati, D.; Andarwulan, N. Screening of In-Vitro Anti-Inflammatory and Antioxidant Activity of Sargassum ilicifolium Crude Lipid Extracts from Different Coastal Areas in Indonesia. Mar. Drugs 2021, 19, 252. https://0-doi-org.brum.beds.ac.uk/10.3390/md19050252
Saraswati, Giriwono PE, Iskandriati D, Andarwulan N. Screening of In-Vitro Anti-Inflammatory and Antioxidant Activity of Sargassum ilicifolium Crude Lipid Extracts from Different Coastal Areas in Indonesia. Marine Drugs. 2021; 19(5):252. https://0-doi-org.brum.beds.ac.uk/10.3390/md19050252
Chicago/Turabian StyleSaraswati, Puspo Edi Giriwono, Diah Iskandriati, and Nuri Andarwulan. 2021. "Screening of In-Vitro Anti-Inflammatory and Antioxidant Activity of Sargassum ilicifolium Crude Lipid Extracts from Different Coastal Areas in Indonesia" Marine Drugs 19, no. 5: 252. https://0-doi-org.brum.beds.ac.uk/10.3390/md19050252