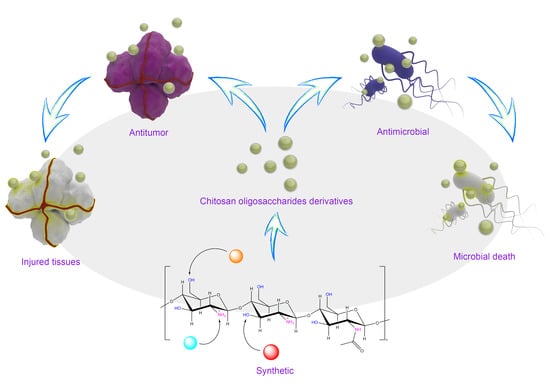
The Microstructure, Antibacterial and Antitumor Activities of Chitosan Oligosaccharides and Derivatives
Abstract
:1. Introduction
2. Synthesis and Structural Attributes of COS
2.1. Key Structural Properties of COSs
2.2. Acylated COS Derivatives
2.3. Alkylated COS Derivatives
2.4. Quaternized COS Derivatives
2.5. Other COS Derivatives
3. Derivation on a Protective Basis
4. Antibacterial Characteristics of COSs
4.1. Mode of Action of COSs as Antibacterial Agents
4.2. Characteristics Affecting Antibacterial Activity
5. Antitumor Activity of COSs
5.1. Antitumor Activity
5.2. Tumor-Targeted Drug Systems
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wan, M.C.; Qin, W.; Lei, C.; Li, Q.H.; Meng, M.; Fang, M.; Song, W.; Chen, J.H.; Tay, F.; Niu, L.N. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Critchley, A.T. The COVID-19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Marangon, C.A.; Martins, V.C.A.; Ling, M.H.; Melo, C.C.; Plepis, A.M.G.; Meyer, R.L.; Nitschke, M. Combination of Rhamnolipid and Chitosan in Nanoparticles Boosts Their Antimicrobial Efficacy. ACS Appl. Mater. Interfaces 2020, 12, 5488–5499. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tondervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Dana, P.M.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B. Chitosan applications in studying and managing osteosarcoma. Int. J. Biol. Macromol. 2021, 169, 321–329. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Li, P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr. Polym. 2019, 222, 115004. [Google Scholar] [CrossRef]
- Manek, E.; Darvas, F.; Petroianu, G.A. Use of Biodegradable, Chitosan-Based Nanoparticles in the Treatment of Alzheimer’s Disease. Molecules 2020, 25, 4866. [Google Scholar] [CrossRef]
- Hao, C.; Wang, W.; Wang, S.; Zhang, L.; Guo, Y. An Overview of the Protective Effects of Chitosan and Acetylated Chitosan Oligosaccharides against Neuronal Disorders. Mar. Drugs 2017, 15, 89. [Google Scholar] [CrossRef] [Green Version]
- Prabaharan, M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. [Google Scholar] [CrossRef]
- Varela-Fernandez, R.; Garcia-Otero, X.; Diaz-Tome, V.; Regueiro, U.; Lopez-Lopez, M.; Gonzalez-Barcia, M.; Lema, M.I.; Otero-Espinar, F.J. Design, Optimization, and Characterization of Lactoferrin-Loaded Chitosan/TPP and Chitosan/Sulfobutylether-beta-cyclodextrin Nanoparticles as a Pharmacological Alternative for Keratoconus Treatment. ACS Appl. Mater. Interfaces 2021, 13, 3559–3575. [Google Scholar] [CrossRef]
- Liu, X.; Hao, M.; Chen, Z.; Zhang, T.; Huang, J.; Dai, J.; Zhang, Z. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials 2021, 272, 120771. [Google Scholar] [CrossRef]
- Shah, B.R.; Li, B.; Al Sabbah, H.; Xu, W.; Mráz, J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends Food Sci. Technol. 2020, 102, 178–192. [Google Scholar] [CrossRef]
- Da Silva Alves, D.C.; Healy, B.; Pinto, L.A.A.; Cadaval, T.R.S., Jr.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Balijepalli, A.S.; Sabatelle, R.C.; Chen, M.; Suki, B.; Grinstaff, M.W. A Synthetic Bioinspired Carbohydrate Polymer with Mucoadhesive Properties. Angew. Chem. Int. Ed. Engl. 2020, 59, 704–710. [Google Scholar] [CrossRef]
- Benchamas, G.; Huang, G.; Huang, S.; Huang, H. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Gomes, A.P.; Varela, A.T.; Duarte, F.V.; Rolo, A.P.; Palmeira, C.M. Hepatic and skeletal muscle mitochondrial toxicity of chitosan oligosaccharides of normal and diabetic rats. Toxicol. Mech. Methods 2016, 26, 650–657. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Sun, Y.; Xing, J.; Yamamoto, A.; Gao, Y. Absorption-improving effects of chitosan oligomers based on their mucoadhesive properties: A comparative study on the oral and pulmonary delivery of calcitonin. Drug Deliv. 2016, 23, 2419–2427. [Google Scholar] [CrossRef]
- Mattaveewong, T.; Wongkrasant, P.; Chanchai, S.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses tumor progression in a mouse model of colitis-associated colorectal cancer through AMPK activation and suppression of NF-kappaB and mTOR signaling. Carbohydr. Polym. 2016, 145, 30–36. [Google Scholar] [CrossRef]
- Schimpf, U.; Nachmann, G.; Trombotto, S.; Houska, P.; Yan, H.; Bjorndahl, L.; Crouzier, T. Assessment of Oligo-Chitosan Biocompatibility toward Human Spermatozoa. ACS Appl. Mater. Interfaces 2019, 11, 46572–46584. [Google Scholar] [CrossRef]
- Sutthasupha, P.; Lungkaphin, A. The potential roles of chitosan oligosaccharide in prevention of kidney injury in obese and diabetic conditions. Food Funct. 2020, 11, 7371–7388. [Google Scholar] [CrossRef]
- Milkova, V. Electrosteric stabilization of oil/water emulsions by adsorption of chitosan oligosaccharides-An electrokinetic study. Carbohydr. Polym. 2021, 265, 118072. [Google Scholar] [CrossRef]
- Liu, X.; Xia, W.; Jiang, Q.; Yu, P.; Yue, L. Chitosan oligosaccharide-N-chlorokojic acid mannich base polymer as a potential antibacterial material. Carbohydr. Polym. 2018, 182, 225–234. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Q.; Xia, W. One-step procedure for enhancing the antibacterial and antioxidant properties of a polysaccharide polymer: Kojic acid grafted onto chitosan. Int. J. Biol. Macromol. 2018, 113, 1125–1133. [Google Scholar] [CrossRef]
- Yue, L.; Sun, D.; Mahmood Khan, I.; Liu, X.; Jiang, Q.; Xia, W. Cinnamyl alcohol modified chitosan oligosaccharide for enhancing antimicrobial activity. Food Chem. 2020, 309, 125513. [Google Scholar] [CrossRef]
- Balijepalli, A.S.; Grinstaff, M.W. Poly-Amido-Saccharides (PASs): Functional Synthetic Carbohydrate Polymers Inspired by Nature. Acc. Chem. Res. 2020, 53, 2167–2179. [Google Scholar] [CrossRef]
- Sahariah, P.; Árnadóttir, B.; Másson, M. Synthetic strategy for selective N-modified and O-modified PEGylated chitosan derivatives. Eur. Polym. J. 2016, 81, 53–63. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Queda, F.; Santos, C.V.; Marques, M.M. Selective Modification of Chitin and Chitosan: En Route to Tailored Oligosaccharides. Chem. Asian J. 2016, 11, 3468–3481. [Google Scholar] [CrossRef]
- Hao, C.; Gao, L.; Zhang, Y.; Wang, W.; Yu, G.; Guan, H.; Zhang, L.; Li, C. Acetylated chitosan oligosaccharides act as antagonists against glutamate-induced PC12 cell death via Bcl-2/Bax signal pathway. Mar. Drugs 2015, 13, 1267–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Liu, M.; Liu, Q.; Wang, W.; Du, Y.; Yin, H. The inhibition of LPS-induced inflammation in RAW264.7 macrophages via the PI3K/Akt pathway by highly N-acetylated chitooligosaccharide. Carbohydr. Polym. 2017, 174, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhu, Y.; Xie, J.; Yin, X. Antioxidant activity of N-acyl chitosan oligosaccharide with same substituting degree. Bioorg. Med. Chem. Lett. 2011, 21, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, J.; Tan, L.; Sun, C.; Dong, J. Synthesis of N-furoyl chitosan and chito-oligosaccharides and evaluation of their antioxidant activity in vitro. Int. J. Biol. Macromol. 2013, 59, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Malekshah, R.E.; Shakeri, F.; Khaleghian, A.; Salehi, M. Developing a biopolymeric chitosan supported Schiff-base and Cu(II), Ni(II) and Zn(II) complexes and biological evaluation as pro-drug. Int. J. Biol. Macromol. 2020, 152, 846–861. [Google Scholar] [CrossRef]
- Antony, R.; Arun, T.; Manickam, S.T.D. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- Huo, M.; Zhang, Y.; Zhou, J.; Zou, A.; Li, J. Formation, microstructure, biodistribution and absence of toxicity of polymeric micelles formed by N-octyl-N,O-carboxymethyl chitosan. Carbohydr. Polym. 2011, 83, 1959–1969. [Google Scholar] [CrossRef]
- Mao, S.; Wang, B.; Yue, L.; Xia, W. Effects of citronellol grafted chitosan oligosaccharide derivatives on regulating anti-inflammatory activity. Carbohydr. Polym. 2021, 262, 117972. [Google Scholar] [CrossRef]
- Mao, S.; Liu, X.; Xia, W. Chitosan oligosaccharide-g-linalool polymer as inhibitor of hyaluronidase and collagenase activity. Int. J. Biol. Macromol. 2021, 166, 1570–1577. [Google Scholar] [CrossRef]
- Yue, L.; Zheng, M.; Wang, M.; Khan, I.M.; Wang, B.; Ma, X.; Peng, C.; Wang, Z.; Xia, W. A general strategy to synthesis chitosan oligosaccharide-O-Terpenol derivatives with antibacterial properties. Carbohydr. Res. 2021, 503, 108315. [Google Scholar] [CrossRef]
- De Almeida, W.S.; da Silva, D.A. Does polysaccharide quaternization improve biological activity? Int. J. Biol. Macromol. 2021, 182, 1419–1436. [Google Scholar] [CrossRef]
- Khaira, G.K.; Kumariya, R.; Chibber, M.; Ghosh, M. Development of a quaternized chitosan with enhanced antibacterial efficacy. J. Water Health 2013, 11, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Tan, Z.; Sun, F.; Sheng, L.; Zhang, X.; Yao, F. Synthesis and characterization of quaternized carboxymethyl chitosan/poly(amidoamine) dendrimer core-shell nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 2026–2036. [Google Scholar] [CrossRef]
- Fu, X.; Shen, Y.; Jiang, X.; Huang, D.; Yan, Y. Chitosan derivatives with dual-antibacterial functional groups for antimicrobial finishing of cotton fabrics. Carbohydr. Polym. 2011, 85, 221–227. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Pang, C.; Luo, J.; Luo, Y.; Sun, R. Preparation and antimicrobial property of chitosan oligosaccharide derivative/rectorite nanocomposite. Carbohydr. Polym. 2013, 92, 1078–1085. [Google Scholar] [CrossRef]
- Tang, F.-X.; Li, H.-C.; Ren, X.-D.; Sun, Y.; Xie, W.; Wang, C.-Y.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D. Preparation and antifungal properties of monosubstituted zinc(Π) phthalocyanine-chitosan oligosaccharide conjugates and their quaternized derivatives. Dye. Pigment. 2018, 159, 439–448. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Hwang, J.W.; Han, Y.K.; Lee, J.S.; Kim, S.K.; Jeon, Y.J.; Moon, S.H.; Jeon, B.T.; Bahk, Y.Y.; et al. Sulfated chitosan oligosaccharides suppress LPS-induced NO production via JNK and NF-kappaB inactivation. Molecules 2014, 19, 18232–18247. [Google Scholar] [CrossRef] [Green Version]
- Je, J.Y.; Kim, E.K.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Kim, B.; Park, T.K.; Park, P.J. Sulfated chitooligosaccharides as prolyl endopeptidase inhibitor. Int. J. Biol. Macromol. 2007, 41, 529–533. [Google Scholar] [CrossRef]
- Lu, Y.; Slomberg, D.L.; Schoenfisch, M.H. Nitric oxide-releasing chitosan oligosaccharides as antibacterial agents. Biomaterials 2014, 35, 1716–1724. [Google Scholar] [CrossRef] [Green Version]
- Reighard, K.P.; Ehre, C.; Rushton, Z.L.; Ahonen, M.J.R.; Hill, D.B.; Schoenfisch, M.H. Role of Nitric Oxide-Releasing Chitosan Oligosaccharides on Mucus Viscoelasticity. ACS Biomater. Sci. Eng. 2017, 3, 1017–1026. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Chen, Y.; Mi, Y.; Tan, W.; Miao, Q.; Li, Q.; Dong, F.; Guo, Z. Preparation of 2,6-diurea-chitosan oligosaccharide derivatives for efficient antifungal and antioxidant activities. Carbohydr. Polym. 2020, 234, 115903. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Hou, Z.; Vikhe, Y.S.; Thappeta, K.R.V.; Marimuthu, K.; De, P.P.; Ng, O.T.; Li, P.; Zhu, Y.; Pethe, K.; et al. Antimicrobial Effect of a Novel Chitosan Derivative and Its Synergistic Effect with Antibiotics. ACS Appl. Mater. Interfaces 2021, 13, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Masson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Gaware, V.S.; Lieder, R.; Jonsdottir, S.; Hjalmarsdottir, M.A.; Sigurjonsson, O.E.; Masson, M. The effect of substituent, degree of acetylation and positioning of the cationic charge on the antibacterial activity of quaternary chitosan derivatives. Mar. Drugs 2014, 12, 4635–4658. [Google Scholar] [CrossRef] [PubMed]
- Benediktsdóttir, B.E.; Gaware, V.S.; Rúnarsson, Ö.V.; Jónsdóttir, S.; Jensen, K.J.; Másson, M. Synthesis of N,N,N-trimethyl chitosan homopolymer and highly substituted N-alkyl-N,N-dimethyl chitosan derivatives with the aid of di-tert-butyldimethylsilyl chitosan. Carbohydr. Polym. 2011, 86, 1451–1460. [Google Scholar] [CrossRef]
- Sahariah, P.; Oskarsson, B.M.; Hjalmarsdottir, M.A.; Masson, M. Synthesis of guanidinylated chitosan with the aid of multiple protecting groups and investigation of antibacterial activity. Carbohydr. Polym. 2015, 127, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Acid hydrolysis of chitosans. Carbohydr. Polym. 2001, 46, 89–98. [Google Scholar] [CrossRef]
- Pasquina-Lemonche, L.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J.; et al. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef]
- Reith, J.; Mayer, C. Peptidoglycan turnover and recycling in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2011, 92, 1–11. [Google Scholar] [CrossRef]
- Neuhaus, F.C.; Baddiley, J. A continuum of anionic charge: Structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 686–723. [Google Scholar] [CrossRef] [Green Version]
- Schneewind, O.; Missiakas, D. Lipoteichoic Acid Synthesis and Function in Gram-Positive Bacteria. In Biogenesis of Fatty Acids, Lipids and Membranes; Springer: Berlin/Heidelberg, Germany, 2019; pp. 163–180. [Google Scholar]
- Yin, J.; Meng, Q.; Cheng, D.; Fu, J.; Luo, Q.; Liu, Y.; Yu, Z. Mechanisms of bactericidal action and resistance of polymyxins for Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 3771–3780. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [Green Version]
- Vila, J.; Moreno-Morales, J.; Balleste-Delpierre, C. Current landscape in the discovery of novel antibacterial agents. Clin. Microbiol. Infect. 2020, 26, 596–603. [Google Scholar] [CrossRef]
- Ali, A.; Noh, N.M.; Mustafa, M.A. Antimicrobial activity of chitosan enriched with lemongrass oil against anthracnose of bell pepper. Food Packag. Shelf Life 2015, 3, 56–61. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Yue, L.; Wang, M.; Khan, I.M.; Niazi, S.; Wang, B.; Ma, X.; Wang, Z.; Xia, W. Preparation and characterization of chitosan oligosaccharide derivatives containing cinnamyl moieties with enhanced antibacterial activities. LWT 2021, 147, 111663. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, J.; Li, R.; Zhou, J.; Wei, J.; Jiao, S.; Wang, Z.A.; Du, Y. Chitosan Oligosaccharides Coupling Inhibits Bacterial Biofilm-Related Antibiotic Resistance against Florfenicol. Molecules 2020, 25, 6043. [Google Scholar] [CrossRef]
- Aytemir, M.D.; Ozcelik, B. A study of cytotoxicity of novel chlorokojic acid derivatives with their antimicrobial and antiviral activities. Eur. J. Med. Chem. 2010, 45, 4089–4095. [Google Scholar] [CrossRef]
- Liu, X.; Xia, W.; Jiang, Q.; Xu, Y.; Yu, P. Synthesis, characterization, and antimicrobial activity of kojic acid grafted chitosan oligosaccharide. J. Agric. Food Chem. 2014, 62, 297–303. [Google Scholar] [CrossRef]
- Lira, M.H.P.d.; Andrade Júnior, F.P.d.; Moraes, G.F.Q.; Macena, G.d.S.; Pereira, F.d.O.; Lima, I.O. Antimicrobial activity of geraniol: An integrative review. J. Essent. Oil Res. 2020, 32, 187–197. [Google Scholar] [CrossRef]
- Bi, R.; Yue, L.; Niazi, S.; Khan, I.M.; Sun, D.; Wang, B.; Wang, Z.; Jiang, Q.; Xia, W. Facile synthesis and antibacterial activity of geraniol conjugated chitosan oligosaccharide derivatives. Carbohydr. Polym. 2021, 251, 117099. [Google Scholar] [CrossRef]
- Chokradjaroen, C.; Theeramunkong, S.; Yui, H.; Saito, N.; Rujiravanit, R. Cytotoxicity against cancer cells of chitosan oligosaccharides prepared from chitosan powder degraded by electrical discharge plasma. Carbohydr. Polym. 2018, 201, 20–30. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Chen, X.; Tian, J.; Li, L.; Zhao, M.; Jiao, Y.; Zhou, C. Effect of chitooligosaccharides on cyclin D1, bcl-xl and bcl-2 mRNA expression in A549 cells using quantitative PCR. Chin. Sci. Bull. 2011, 56, 1629–1632. [Google Scholar] [CrossRef] [Green Version]
- Zhai, X.; Li, C.; Ren, D.; Wang, J.; Ma, C.; Abd El-Aty, A.M. The impact of chitooligosaccharides and their derivatives on the in vitro and in vivo antitumor activity: A comprehensive review. Carbohydr. Polym. 2021, 266, 118132. [Google Scholar] [CrossRef]
- Huang, R.; Mendis, E.; Rajapakse, N.; Kim, S.K. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2006, 78, 2399–2408. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, S.; Hou, J.; Chi, J.; Wang, S.; Shao, K.; Liu, W.; Sun, R.; Han, B. Effects of carboxymethyl chitosan oligosaccharide on regulating immunologic function and inhibiting tumor growth. Carbohydr. Polym. 2020, 250, 116994. [Google Scholar] [CrossRef]
- Ignjatovic, N.L.; Sakac, M.; Kuzminac, I.; Kojic, V.; Markovic, S.; Vasiljevic-Radovic, D.; Wu, V.M.; Uskokovic, V.; Uskokovic, D.P. Chitosan Oligosaccharide Lactate Coated Hydroxyapatite Nanoparticles as a Vehicle for the Delivery of Steroid Drugs and the Targeting of Breast Cancer Cells. J. Mater. Chem. B 2018, 6, 6957–6968. [Google Scholar] [CrossRef] [Green Version]
- Jadidi-Niaragh, F.; Atyabi, F.; Rastegari, A.; Kheshtchin, N.; Arab, S.; Hassannia, H.; Ajami, M.; Mirsanei, Z.; Habibi, S.; Masoumi, F.; et al. CD73 specific siRNA loaded chitosan lactate nanoparticles potentiate the antitumor effect of a dendritic cell vaccine in 4T1 breast cancer bearing mice. J. Control. Release 2017, 246, 46–59. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, J.; Fu, Y.; Zheng, Y.; Shen, W.; Zhou, J.; Yin, T. Deeply Infiltrating iRGD-Graphene Oxide for the Intensive Treatment of Metastatic Tumors through PTT-Mediated Chemosensitization and Strengthened Integrin Targeting-Based Antimigration. Adv. Healthc. Mater. 2021, 10, e2100536. [Google Scholar] [CrossRef]
- Lee, S.; Jung, J.S.; Jo, G.; Yang, D.H.; Koh, Y.S.; Hyun, H. Near-Infrared Fluorescent Sorbitol Probe for Targeted Photothermal Cancer Therapy. Cancers 2019, 11, 1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Li, Q.; Liu, R.; Zhang, X.; Li, Z.; Luan, Y. A Versatile Prodrug Strategy to In Situ Encapsulate Drugs in MOF Nanocarriers: A Case of Cytarabine-IR820 Prodrug Encapsulated ZIF-8 toward Chemo-Photothermal Therapy. Adv. Funct. Mater. 2018, 28, 1802830. [Google Scholar] [CrossRef]
- Lee, S.; Jo, G.; Jung, J.S.; Yang, D.H.; Hyun, H. Near-infra-red fluorescent chitosan oligosaccharide lactate for targeted cancer imaging and photothermal therapy. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Tarapcsak, S.; Gyongy, Z.; Ritter, Z.; Batta, G.; Bosire, R.; Remenyik, J.; Goda, K. Effects of Polyphenols on P-Glycoprotein (ABCB1) Activity. Pharmaceutics 2021, 13, 2062. [Google Scholar] [CrossRef]
- Liang, L.; Huo, W.; Wang, B.; Cao, L.; Huo, H.; Liu, Y.; Jin, Y.; Yang, X. DNAzyme-Based nanoflowers for reversing P-glycoprotein-mediated multidrug resistance in breast cancer. J. Colloid Interface Sci. 2022, 608, 2985–2993. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of Cancer Drug Resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Anderson, R.C.; Lan, X.; Conti, P.S.; Chen, K. Recent advances in the development of nanoparticles for multimodality imaging and therapy of cancer. Med. Res. Rev. 2020, 40, 909–930. [Google Scholar] [CrossRef]
- Hu, F.Q.; Chen, W.W.; Zhao, M.D.; Yuan, H.; Du, Y.Z. Effective antitumor gene therapy delivered by polyethylenimine-conjugated stearic acid-g-chitosan oligosaccharide micelles. Gene Ther. 2013, 20, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Chae, S.Y.; Son, S.; Lee, M.; Jang, M.K.; Nah, J.W. Deoxycholic acid-conjugated chitosan oligosaccharide nanoparticles for efficient gene carrier. J. Control. Release 2005, 109, 330–344. [Google Scholar] [CrossRef]
- Jia, L.; Li, Z.; Zheng, D.; Li, Z.; Zhao, Z. A targeted and redox/pH-responsive chitosan oligosaccharide derivatives based nanohybrids for overcoming multidrug resistance of breast cancer cells. Carbohydr. Polym. 2021, 251, 117008. [Google Scholar] [CrossRef]
- Lee, J.Y.; Termsarasab, U.; Lee, M.Y.; Kim, D.H.; Lee, S.Y.; Kim, J.S.; Cho, H.J.; Kim, D.D. Chemosensitizing indomethacin-conjugated chitosan oligosaccharide nanoparticles for tumor-targeted drug delivery. Acta Biomater. 2017, 57, 262–273. [Google Scholar] [CrossRef]
- Zhan, X.; Teng, W.; Sun, K.; He, J.; Yang, J.; Tian, J.; Huang, X.; Zhou, L.; Zhou, C. CD47-mediated DTIC-loaded chitosan oligosaccharide-grafted nGO for synergistic chemo-photothermal therapy against malignant melanoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112014. [Google Scholar] [CrossRef]
- Chen, X.; Bremner, D.H.; Ye, Y.; Lou, J.; Niu, S.; Zhu, L.-M. A dual-prodrug nanoparticle based on chitosan oligosaccharide for enhanced tumor-targeted drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126512. [Google Scholar] [CrossRef]
- Jin, R.; Wang, J.; Gao, M.; Zhang, X. Pollen-like silica nanoparticles as a nanocarrier for tumor targeted and pH-responsive drug delivery. Talanta 2021, 231, 122402. [Google Scholar] [CrossRef]
- Meng, X.; Jia, K.; Sun, K.; Zhang, L.; Wang, Z. Smart responsive nanoplatform via in situ forming disulfiram-copper ion chelation complex for cancer combination chemotherapy. Chem. Eng. J. 2021, 415, 128974. [Google Scholar] [CrossRef]
- Pu, X.Q.; Ju, X.J.; Zhang, L.; Cai, Q.W.; Liu, Y.Q.; Peng, H.Y.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.Y. Novel Multifunctional Stimuli-Responsive Nanoparticles for Synergetic Chemo-Photothermal Therapy of Tumors. ACS Appl. Mater. Interfaces 2021, 13, 28802–28817. [Google Scholar] [CrossRef]
- Chen, K.; Qian, Y.; Wang, C.; Yang, D.; Qiu, X.; Binks, B.P. Tumor microenvironment-responsive, high internal phase Pickering emulsions stabilized by lignin/chitosan oligosaccharide particles for synergistic cancer therapy. J. Colloid Interface Sci. 2021, 591, 352–362. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Feng, J.; You, H.; Zhou, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Microstructure, Antibacterial and Antitumor Activities of Chitosan Oligosaccharides and Derivatives. Mar. Drugs 2022, 20, 69. https://0-doi-org.brum.beds.ac.uk/10.3390/md20010069
Yu D, Feng J, You H, Zhou S, Bai Y, He J, Cao H, Che Q, Guo J, Su Z. The Microstructure, Antibacterial and Antitumor Activities of Chitosan Oligosaccharides and Derivatives. Marine Drugs. 2022; 20(1):69. https://0-doi-org.brum.beds.ac.uk/10.3390/md20010069
Chicago/Turabian StyleYu, Dawei, Jiayao Feng, Huimin You, Shipeng Zhou, Yan Bai, Jincan He, Hua Cao, Qishi Che, Jiao Guo, and Zhengquan Su. 2022. "The Microstructure, Antibacterial and Antitumor Activities of Chitosan Oligosaccharides and Derivatives" Marine Drugs 20, no. 1: 69. https://0-doi-org.brum.beds.ac.uk/10.3390/md20010069