Extraction, Characterization and Osteogenic Activity of a Type I Collagen from Starfish (Asterias amurensis)
Abstract
:1. Introduction
2. Results
2.1. Protein Pattern of Collagen
2.2. Amino Acid Composition of AAC
2.3. Characteristic Absorption of AAC
2.3.1. UV Spectral Properties
2.3.2. FTIR Spectral Properties
2.3.3. Circular Dichroism (CD) Spectral Properties
2.4. Thermal Stability
2.5. Microstructural Analysis
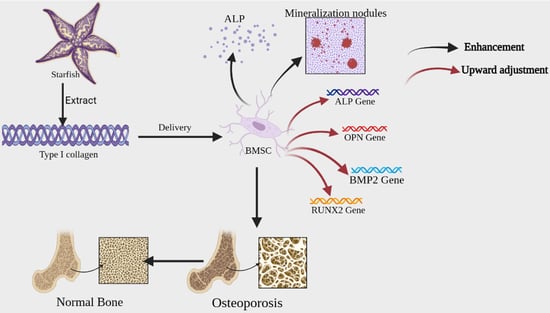
2.6. AAC Promotes BMSCs Proliferation
2.7. AAC Enhances ALP Activity
2.8. AAC Promotes Calcified Nodules in BMSCs
2.9. AAC Upregulates mRNA Expression of Osteogenic Genes
3. Discussion
3.1. SDS-PAGE
3.2. Analysis of Amino Acid Composition
3.3. Characteristic Absorption
3.4. Osteogenic Activity Analysis
4. Materials and Methods
4.1. Reagents
4.2. Raw Materials and Pre-Treatment
4.3. Extraction of AAC
4.4. Amino Acid Composition
4.5. Sodium Dodecyl Sulfonate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.6. Ultraviolet (UV) Scanning Analysis
4.7. Fourier-Transform Infrared (FTIR) Spectroscopy
4.8. CD Spectral
4.9. Scanning Electron Microscopy (SEM)
4.10. Differential scanning Calorimetry (DSC)
4.11. Cell Culture
4.12. Proliferation Assay
4.13. Alkaline Phosphatase (ALP) Activity
4.14. Calcified Cell Nodules
4.15. RT-PCR Detection of Genes Related to Osteogenic Differentiation in Mice
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeff Ross, D.; Johnson, C.R.; Hewitt, C.L. Variability in the impact of an introduced predator (Asterias amurensis: Asteroidea) on soft-sediment assemblages. J. Exp. Mar. Biol. Ecol. 2003, 288, 257–278. [Google Scholar] [CrossRef]
- Brodie, J.; Fabricius, K.; De’ath, G.; Okaji, K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. Pollut. Bull. 2005, 51, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, R.E.; Bradbury, R.H.; Moran, P.J. The crown-of-thorns starfish, Acanthaster planci, on the great barrier reef. Math. Comput. Model. 1990, 13, 45–60. [Google Scholar] [CrossRef]
- Ross, D.J.; Johnson, C.R.; Hewitt, C.L.J.B.I. Assessing the ecological impacts of an introduced seastar: The importance of multiple methods. Biol. Invasions 2003, 5, 3–21. [Google Scholar] [CrossRef]
- Hatanaka, M.; Kosaka, M. Biological studies on the population of the starfish, Asterias amurencis, in Sendai Bay. Tohoku J. Agr. Res. 1959, 9, 159–178. [Google Scholar]
- Nojima, S.; Soliman, F.E.; Kondo, Y.; Kuwano, Y.; Nasu, K.; Kitajima, C. Some notes of the outbreak of the sea star Asterias amurensis versiclor Sladen, in the Ariake Sea, western Kyshu. Publ. Amakusa Mar. Biol. Lab. Kyushu Univ. 1986, 8, 89–112. [Google Scholar]
- Fraser, N.; Crawford, B.R.; Kusen, J.; Indonesia, P.P.C. Best Practices Guide for Crown-of-Thorns Clean-Ups; Coastal Resources Center, University of Rhode Island Narragansett: Kingston, RI, USA, 2000; Volume 2225. [Google Scholar]
- Mah, C.L.; Blake, D.B. Global diversity and phylogeny of the Asteroidea (Echinodermata). PLoS ONE 2012, 7, e35644. [Google Scholar] [CrossRef]
- Grannum, R.; Murfet, N.; Ritz, D.; Turner, E. The Distribution and Impact of the Exotic Seastar, Asterias amurensis (Lutken) in Tasmania; University of Tasmania: Hobart, Australia, 1996. [Google Scholar]
- Malyarenko, T.V.; Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Popov, R.S.; Vishchuk, O.S.; Stonik, V.A. Asterosaponins from the Far Eastern starfish Leptasterias ochotensis and their anticancer activity. Steroids 2014, 87, 119–127. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of Brownstripe red snapper (Lutjanus vitta). Food Chem. 2005, 93, 475–484. [Google Scholar] [CrossRef]
- Kozlowska, J.; Sionkowska, A.; Skopinska-Wisniewska, J.; Piechowicz, K. Northern pike (Esox lucius) collagen: Extraction, characterization and potential application. Int. J. Biol. Macromol. 2015, 81, 220–227. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.-d.A. Marine shells: Potential opportunities for extraction of functional and health-promoting materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Matsumura, T. Shape, size and amino acid composition of collagen fibril of the starfish Asterias amurensis. Comp. Biochem. Physiol. Part B Comp. Biochem. 1973, 44, 1197–1205. [Google Scholar] [CrossRef]
- Lee, K.-j.; Park, H.Y.; Kim, Y.K.; Park, J.I.; Yoon, H.D. Biochemical Characterization of Collagen from the Starfish Asterias amurensis. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 221–226. [Google Scholar] [CrossRef]
- Li, Z.J.; Kim, S.M. Structural Identification and Proteolytic Effects of the Hatching Enzyme from Starfish Asterias amurensis. Protein Pept. Lett. 2014, 21, 631–638. [Google Scholar] [CrossRef]
- Tan, C.; Karim, A.; Latiff, A.; Gan, C.; Ghazali, F. Extraction and characterization of pepsin-solubilized collagen from the body wall of crown-of-thorns Starfish (Acanthaster planci). Int. Food Res. J. 2013, 20, 3013–3020. [Google Scholar]
- Kumar Vate, N.; Pawel Strachowski, P.; Undeland, I.; Abdollahi, M. Structural and functional properties of collagen isolated from lumpfish and starfish using isoelectric precipitation vs salting out. Food Chem. X 2023, 18, 100646. [Google Scholar] [CrossRef]
- Vate, N.K.; Undeland, I.; Abdollahi, M. Resource efficient collagen extraction from common starfish with the aid of high shear mechanical homogenization and ultrasound. Food Chem. 2022, 393, 133426. [Google Scholar] [CrossRef]
- Stoilov, I.; Starcher, B.C.; Mecham, R.P.; Broekelmann, T.J. Measurement of elastin, collagen, and total protein levels in tissues. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 143, pp. 133–146. [Google Scholar]
- Kaewdang, O.; Benjakul, S.; Kaewmanee, T.; Kishimura, H. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem. 2014, 155, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Hou, C.; Bao, B.; Pan, Z.; de Val, J.E.M.S.; Elango, J.; Wu, W. Comparison of Physicochemical and Structural Properties of Acid-Soluble and Pepsin-Soluble Collagens from Blacktip Reef Shark Skin. Mar. Drugs 2022, 20, 376. [Google Scholar] [CrossRef]
- Song, X.; Si, L.; Sun, X.; Zhu, X.; Li, Z.; Li, Y.; Wang, Y.; Hou, H. Rheological properties, thermal stability and conformational changes of collagen from sea cucumber (Apostichopus japonicas). Food Chem. 2022, 389, 133033. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-F.; Shi, W.-G.; Zhou, J.; Gao, Y.-H.; Li, S.-F.; Fang, Q.-Q.; Wang, M.-G.; Ma, H.-P.; Wang, J.-F.; Xian, C.J.; et al. Pulsed electromagnetic fields stimulate osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPRII localized at the base of primary cilium. Bone 2016, 93, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Ito-Kato, E.; Suzuki, N.; Nakayama, A.; Ogiso, B.; Maeno, M.; Ito, K. IL-1α affects mineralized nodule formation by rat osteoblasts. Life Sci. 2004, 75, 2317–2327. [Google Scholar] [CrossRef]
- Gao, S.; Chen, B.; Zhu, Z.; Du, C.; Zou, J.; Yang, Y.; Huang, W.; Liao, J. PI3K-Akt signaling regulates BMP2-induced osteogenic differentiation of mesenchymal stem cells (MSCs): A transcriptomic landscape analysis. Stem Cell Res. 2023, 66, 103010. [Google Scholar] [CrossRef]
- Charoensin, S.; Pothacharoen, P.; Wanachewin, O.; Kongtawelert, P.; Suttajit, M. Chapter 15—Functional foods in improving bone health during aging. In Plant Bioactives as Natural Panacea against Age-Induced Diseases; Pandey, K.B., Suttajit, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 287–305. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus). Food Sci. Technol. 2010, 43, 792–800. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Luo, X.; Liu, W.; Zhao, M.; Liu, T.; Xiong, F.; Lei, L.; Jia, F.; Feng, F. A novel Atlantic salmon (Salmo salar) bone collagen peptide delays osteoarthritis development by inhibiting cartilage matrix degradation and anti-inflammatory. Food Res. Int. 2022, 162, 112148. [Google Scholar] [CrossRef]
- Mallick, M.; Are, R.P.; Babu, A.R. An overview of collagen/bioceramic and synthetic collagen for bone tissue engineering. Materialia 2022, 101391. [Google Scholar] [CrossRef]
- Licini, C.; Vitale-Brovarone, C.; Mattioli-Belmonte, M. Collagen and non-collagenous proteins molecular crosstalk in the pathophysiology of osteoporosis. Cytokine Growth Factor Rev. 2019, 49, 59–69. [Google Scholar] [CrossRef] [PubMed]






| Amino Acid | Starfish Collagen |
|---|---|
| Aspartic acid (Asp) | 82.51 ± 0.92 |
| Threonine (Thr) | 32.36 ± 0.37 |
| Serine (Ser) | 68.66 ± 1.51 |
| Glutamic acid (Glu) | 89.42 ± 0.25 |
| Glycine (Gly) | 297.43 ± 0.58 |
| Alanine (Ala) | 98.81 ± 3.59 |
| Valine (Val) | 21.98 ± 0.37 |
| Isoleucine (Ile) | 15.06 ± 0.38 |
| Leucine (Leu) | 16.30 ± 0.38 |
| Tyrosine (Tyr) | 8.40 ± 0.38 |
| Phenylalanine (Phe) | 8.89 ± 0.38 |
| Lysine (Lys) | 25.94 ± 0.21 |
| Histidine (His) | 2.22 ± 0.2 |
| Arginine (Arg) | 45.95 ± 0.42 |
| Proline (Pro) | 86.22 ± 0.25 |
| Hydroxyproline (Hyp) | 99.80 ± 0,37 |
| Cysteine (Cys) | 0 |
| Total | 1000 |
| Gene | PCR Primer | |
|---|---|---|
| F | R | |
| GAPDH | 5′-AATCCCATCACCATCTTCC-3′ | 5′-GCAGAGATGATGACCCTTT-3′ |
| ALP | CACGGCGTCCATGAGCAGAAC | CAGGCACAGTGGTCAAGGTTGG |
| RUNX-2 | CGGCAAGATGAGCGACGTGAG | GCTGTTGTTGCTGCTGCTGTTG |
| OPN | ATGGACGACGATGATGACGATGATG | CTTGTGTACTAGCAGTGACGGTCTC |
| BMP2 | AAGCGTCAAGCCAAACACAAACAG | GAGGTGCCACGATCCAGTCATTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Yu, Y.; Wu, W.; Wang, P. Extraction, Characterization and Osteogenic Activity of a Type I Collagen from Starfish (Asterias amurensis). Mar. Drugs 2023, 21, 274. https://0-doi-org.brum.beds.ac.uk/10.3390/md21050274
Li L, Yu Y, Wu W, Wang P. Extraction, Characterization and Osteogenic Activity of a Type I Collagen from Starfish (Asterias amurensis). Marine Drugs. 2023; 21(5):274. https://0-doi-org.brum.beds.ac.uk/10.3390/md21050274
Chicago/Turabian StyleLi, Lingcui, Yu Yu, Wenhui Wu, and Peipei Wang. 2023. "Extraction, Characterization and Osteogenic Activity of a Type I Collagen from Starfish (Asterias amurensis)" Marine Drugs 21, no. 5: 274. https://0-doi-org.brum.beds.ac.uk/10.3390/md21050274