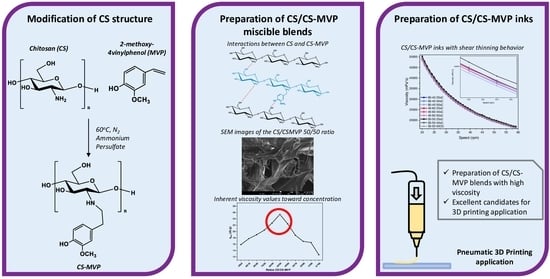
Exploring the Blends’ Miscibility of a Novel Chitosan Derivative with Enhanced Antioxidant Properties; Prospects for 3D Printing Biomedical Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. CS-MVP Derivatives
2.2. CS/CS-MVP Blends
2.3. CS/CS-MVP Inks
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CS-MVP
3.3. Preparation of CS/CS-MVP Blends
3.4. Preparation of CS/MVP Inks
3.5. Characterization Techniques
3.5.1. Fourier-Transformed Infrared Spectroscopies
3.5.2. Nuclear Magnetic Resonance (1H-NMR)
3.5.3. Wide-Angle X-ray Scattering (XRD)
3.5.4. Scanning Electron Microscopy (SEM)
3.5.5. Degree of Substitution
3.5.6. Contact Angle
3.5.7. Swelling and Water Content Capacity
3.5.8. Solubility Measurements
3.5.9. Viscosity Measurements
3.5.10. Viscous Synergy
3.5.11. Miscibility Study
3.5.12. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Titopoulou, A.; Koukaras, E.N.; Kostoglou, M.; Koutris, E.; Karavas, E.; Bikiaris, D.N. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int. J. Pharm. 2015, 495, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Bikiaris, D.N.; Lazaridis, N.K. Low-swelling chitosan derivatives as biosorbents for basic dyes. Langmuir 2008, 24, 4791–4799. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its derivatives for ocular delivery formulations: Recent advances and developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Seidi, F.; Khodadadi Yazdi, M.; Jouyandeh, M.; Dominic, M.; Naeim, H.; Nezhad, M.N.; Bagheri, B.; Habibzadeh, S.; Zarrintaj, P.; Saeb, M.R.; et al. Chitosan-based blends for biomedical applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Aisyah, H.A.; Nordin, A.H.; Ngadi, N.; Yusoff, M.; Zuhri, M.; Rizal, M.; Asyraf, M.; Sapuan, S.M.; Zainudin, E.S.; et al. Nanocomposites for Various Advanced Applications. Polymers 2022, 14, 1–36. [Google Scholar]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef]
- Michailidou, G.; Koukaras, E.N.; Bikiaris, D.N. Vanillin chitosan miscible hydrogel blends and their prospects for 3D printing biomedical applications. Int. J. Biol. Macromol. 2021, 192, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Osi, A.R.; Zhang, H.; Chen, J.; Zhou, Y.; Wang, R.; Fu, J.; Müller-Buschbaum, P.; Zhong, Q. Three-Dimensional-Printable Thermo/Photo-Cross-Linked Methacrylated Chitosan-Gelatin Hydrogel Composites for Tissue Engineering. ACS Appl. Mater. Interfaces 2021, 13, 22902–22913. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Abdel-Hay, F.I.; Tamer, T.M.; Abo-Elghit Ibrahim, E.M.; Mohy Eldin, M.S. Antimicrobial activity of novel modified aminated chitosan with aromatic esters. Polym. Bull. 2020, 77, 1631–1647. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.I.; Go, B.; Oh, U.H.; Kim, C.S.; Jung, Y.H.; Lee, J.; Kim, J.H. 2-Methoxy-4-vinylphenol attenuates migration of human pancreatic cancer cells via blockade of FAK and AKT signaling. Anticancer Res. 2019, 39, 6685–6691. [Google Scholar] [CrossRef]
- Jeong, J.B.; Hong, S.C.; Jeong, H.J.; Koo, J.S. Anti-inflammatory effect of 2-methoxy-4-vinylphenol via the suppression of NF-κB and MAPK activation, and acetylation of histone H3. Arch. Pharm. Res. 2011, 34, 2109–2116. [Google Scholar] [CrossRef]
- Rubab, M.; Chelliah, R.; Saravanakumar, K. Bioactive Potential of 2-Methoxy-4-vinylphenol and. Foods 2020, 9, 568. [Google Scholar] [CrossRef]
- Corrêa, C.C.; Santhiago, M.; de Carvalho Castro Silva, C.; Barboza Formiga, A.L.; Kubota, L.T. Synthesis and electrochemical characterization of poly(2-methoxy-4-vinylphenol) with MWCNTs. Electroanalysis 2011, 23, 2562–2568. [Google Scholar] [CrossRef]
- Ito, K.; Kodaira, K.; Onishi, Y. Copolymerization of 2-Methoxy-4-Vinylphenol with Fluorinated Acrylic Monomers: Water-Repellent Properties of Copolymer. J. Appl. Polym. Sci. 1981, 26, 423–429. [Google Scholar] [CrossRef]
- Langos, D.; Granvogl, M. Studies on the Simultaneous Formation of Aroma-Active and Toxicologically Relevant Vinyl Aromatics from Free Phenolic Acids during Wheat Beer Brewing. J. Agric. Food Chem. 2016, 64, 2325–2332. [Google Scholar] [CrossRef]
- Hussein, H.J.; Hadi, M.Y.; Hameed, I.H. Study of chemical composition of Foeniculum vulgare using Fourier transform infrared spectrophotometer and gas chromatography—Mass spectrometry. J. Pharmacogn. Phyther. 2016, 8, 60–89. [Google Scholar] [CrossRef] [Green Version]
- Ainali, N.M.; Xanthopoulou, E.; Michailidou, G.; Zamboulis, A.; Bikiaris, D.N. Microencapsulation of fluticasone propionate and salmeterol xinafoate in modified chitosan microparticles for release optimization. Molecules 2020, 25, 3888. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Pelton, R. The synthesis of poly(3,4-dihydroxystyrene) and poly [(sodium 4-styrenesulfonate)-co-(3,4-dihydroxystyrene)]. Macromol. Rapid Commun. 1998, 246, 241–246. [Google Scholar] [CrossRef]
- Semwal, L.O.K.; Singh, B.K.; Archana, D.; Verma, A.; Dutta, P.K. Macromolecular chitosan/ciprofloxacin pro-drugs: Synthesis, physico- chemicaland biological assessment for drug delivery systems. J. Polym. Mater. 2012, 29, 1–13. [Google Scholar]
- Chen, W.P.; Huang, D.J.; Hu, Z.; Zhuang, Y.L.; Lu, S.T. Preparation and characterization of 6-O-caffeic acid chitosan. J. Phys. Conf. Ser. 2021, 1765, 012029. [Google Scholar] [CrossRef]
- Rohindra, D.R.; Nand, A.V.; Khurma, J.R. Swelling properties of chitosan hydrogels. South Pac. J. Nat. Appl. Sci. 2004, 22, 32–35. [Google Scholar] [CrossRef]
- Michailidou, G.; Christodoulou, E.; Nanaki, S.; Barmpalexis, P.; Karavas, E.; Vergkizi-Nikolakaki, S.; Bikiaris, D.N. Super-hydrophilic and high strength polymeric foam dressings of modified chitosan blends for topical wound delivery of chloramphenicol. Carbohydr. Polym. 2019, 208, 1–13. [Google Scholar] [CrossRef]
- Palusiak, M.; Grabowski, S.J. Methoxy group as an acceptor of proton in hydrogen bonds. J. Mol. Struct. 2002, 642, 97–104. [Google Scholar] [CrossRef]
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew. Chem. Int. Ed. Engl. 2003, 42, 1210–1250. [Google Scholar] [CrossRef] [PubMed]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the factors affecting the solubility of chitosan in water. Macromol. Chem. Phys. 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, W. Dissolution and stability of chitosan in a sodium hydroxide/urea aqueous solution. J. Appl. Polym. Sci. 2014, 131, 39819. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. ISBN 978-3-642-34243-1. [Google Scholar]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Marques, S.C.; Nunes, P.M.; Pedrosa, R.; Leandro, S.M. Antifungal and antioxidant properties of chitosan polymers obtained from nontraditional Polybius henslowii sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Cruz, M.R.; Villanueva-Carvajal, A.; Morales Rosales, E.J.; Ramírez Dávila, J.F.; Dominguez-Lopez, A. Controlled release and antioxidant activity of Roselle (Hibiscus sabdariffa L.) extract encapsulated in mixtures of carboxymethyl cellulose, whey protein, and pectin. Lwt 2013, 50, 554–561. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Ahmad, Z.; Akil, H.M. X-ray diffraction studies of cross linked chitosan with different cross linking agents for waste water treatment application. AIP Conf. Proc. 2009, 1202, 106–111. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Nanaki, S.; Karavas, E.; Kalantzi, L.; Bikiaris, D. Miscibility study of carrageenan blends and evaluation of their effectiveness as sustained release carriers. Carbohydr. Polym. 2010, 79, 1157–1167. [Google Scholar] [CrossRef]
- Chee, K.K. Determination of polymer-polymer miscibility by viscometry. Eur. Polym. J. 1990, 26, 423–426. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, W.; Feng, Z. Criterion of polymer-polymer miscibility determined by viscometry. Eur. Polym. J. 1992, 28, 1259–1261. [Google Scholar] [CrossRef]
- Lewandowska, K. Viscometric studies in dilute solution mixtures of chitosan and microcrystalline chitosan with poly(vinyl alcohol). J. Solut. Chem. 2013, 42, 1654–1662. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, W.P.; Duarte, J.; Tessaro, I.C.; Baldasso, C. Non-Supported and PET-Supported Chitosan Membranes for Pervaporation: Production, Characterization, and Performance. Membranes 2022, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, G.; Bikiaris, D.N. Novel 3D-Printed Dressings of Chitosan–Vanillin-Modified Chitosan Blends Loaded with Fluticasone Propionate for Treatment of Atopic Dermatitis. Pharmaceutics 2022, 14, 1966. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.N.; Teixeira, V.G.; Delpech, M.C.; Souza, J.V.S.; Costa, M.A.S. Viscometric study of chitosan solutions in acetic acid/sodium acetate and acetic acid/sodium chloride. Carbohydr. Polym. 2015, 133, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peniche, C.; Argüelles-Monal, W. Overview on structural characterization of chitosan molecules in relation with their behavior in solution. Macromol. Symp. 2001, 168, 1–20. [Google Scholar] [CrossRef]
- Joohyang, P.; Kim, D. Effect of Polymer Solution Concentration on the Swelling and Mechanical Properties of Glycol Chitosan Superporous Hydrogels. J. Appl. Polym. Sci. 2010, 115, 3434–3441. [Google Scholar] [CrossRef]
- Desbrieres, J. Viscosity of semiflexible chitosan solutions: Influence of concentration, temperature, and role of intermolecular interactions. Biomacromolecules 2002, 3, 342–349. [Google Scholar] [CrossRef]
- Heidenreich, A.C.; Pérez-Recalde, M.; González Wusener, A.; Hermida, É.B. Collagen and chitosan blends for 3D bioprinting: A rheological and printability approach. Polym. Test. 2020, 82, 106297. [Google Scholar] [CrossRef]
- Ning, L.; Zhu, N.; Mohabatpour, F.; Sarker, M.D.; Schreyer, D.J.; Chen, X. Bioprinting Schwann cell-laden scaffolds from low-viscosity hydrogel compositions. J. Mater. Chem. B 2019, 7, 4538–4551. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination of degree of deacetylation of chitosan—Comparision of methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 2012, 5–20. [Google Scholar]
- Pradhan, P.; Roy, M.N. Viscous synergy and antagonism, excess molar volume, isoentropic compressibility and excess molar refraction of ternary mixtures containing tetrahydrofuran, methanol and six membered cyclic compounds at 298.15K. Phys. Chem. Liq. 2011, 49, 286–301. [Google Scholar] [CrossRef]









| Sample | Degree of Substitution (%) |
|---|---|
| CS-MVP 4-1 | 10.2 |
| CS-MVP 2-1 | 13.6 |
| CS-MVP 1-1 | 14.7 |
| CS-MVP 1-2 | 15.4 |
| Sample | Contact Angle, θ (°) | |
|---|---|---|
| CS | 74.5 |  |
| CS-MVP 4-1 | 73.5 |  |
| CS-MVP 2-1 | 67.8 |  |
| CS-MVP 1-1 | 58 |  |
| CS-MVP 1-2 | 82.6 |  |
| CS (gr) | MVP (μL) | Ammonium Persulfate (mg) | |
|---|---|---|---|
| CS-MVP 4-1 | 1 | 101 | 2 |
| CS-MVP 2-1 | 1 | 203 | 2 |
| CS-MVP 1-1 | 1 | 406 | 2 |
| CS-MVP 1-2 | 1 | 812 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michailidou, G.; Zamboulis, A.; Bikiaris, D.N. Exploring the Blends’ Miscibility of a Novel Chitosan Derivative with Enhanced Antioxidant Properties; Prospects for 3D Printing Biomedical Applications. Mar. Drugs 2023, 21, 370. https://0-doi-org.brum.beds.ac.uk/10.3390/md21070370
Michailidou G, Zamboulis A, Bikiaris DN. Exploring the Blends’ Miscibility of a Novel Chitosan Derivative with Enhanced Antioxidant Properties; Prospects for 3D Printing Biomedical Applications. Marine Drugs. 2023; 21(7):370. https://0-doi-org.brum.beds.ac.uk/10.3390/md21070370
Chicago/Turabian StyleMichailidou, Georgia, Alexandra Zamboulis, and Dimitrios N. Bikiaris. 2023. "Exploring the Blends’ Miscibility of a Novel Chitosan Derivative with Enhanced Antioxidant Properties; Prospects for 3D Printing Biomedical Applications" Marine Drugs 21, no. 7: 370. https://0-doi-org.brum.beds.ac.uk/10.3390/md21070370