Stable Allometric Trajectories in Picea abies (L.) Karst. Trees along an Elevational Gradient
Abstract
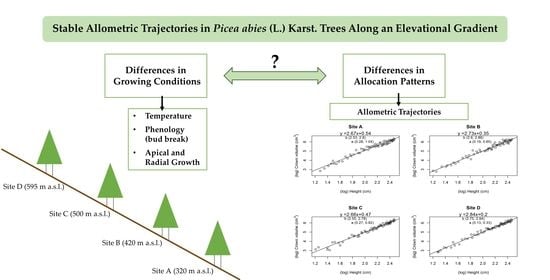
:1. Introduction
- The influence of temperature on bud break and tree growth in order to quantify differences in the growing conditions along the gradient;
- The stability of allometric trajectories with ontogenesis along the gradient.
2. Materials and Methods
2.1. Experimental Set
2.2. Local Performance
- Apical bud break process, measured every two days from 18 May to 5 June 2018;
- Apical shoot elongation, measured every three weeks from 23 May to 4 September 2018;
- Diameter at 20 cm from root collar, measured every three weeks from 23 May to 4 September 2018.
2.3. Allometric Relationships
2.4. Statistical Analyses
3. Results
3.1. Local Performance
3.2. Allometric Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hänninen, H.; Tanino, K. Tree seasonality in a warming climate. Trends Plant Sci. 2011, 16, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Junttila, O. Regulation of annual shoot growth cycle in northern tree species. In Physiology of Northern Plants under Changing Environment; Research Signpost: Kerala, India, 2007; pp. 177–210. [Google Scholar]
- Olsen, J.E. Light and temperature sensing and signaling in induction of bud dormancy in woody plants. Plant Mol. Biol. 2010, 73, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Strømme, C.B.; Julkunen-Tiitto, R.; Krishna, U.; Lavola, A.; Olsen, J.E.; Nybakken, L. UV-B and temperature enhancement affect spring and autumn phenology in Populus tremula: Climate change effects on tree phenology. Plant Cell Environ. 2015, 38, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Basler, D. Phenology under Global Warming. Science 2010, 327, 1461–1462. [Google Scholar] [CrossRef] [PubMed]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Khanduri, V.P.; Sharma, C.M.; Singh, S.P. The effects of climate change on plant phenology. Environmentalist 2008, 28, 143–147. [Google Scholar] [CrossRef]
- Morin, X.; Lechowicz, M.J.; Augspurger, C.; O’keefe, J.; Viner, D.; Chuine, I. Leaf phenology in 22 North American tree species during the 21st century. Glob. Chang. Biol. 2009, 15, 961–975. [Google Scholar] [CrossRef]
- Jyske, T.; Mäkinen, H.; Kalliokoski, T.; Nöjd, P. Intra-annual tracheid production of Norway spruce and Scots pine across a latitudinal gradient in Finland. Agric. For. Meteorol. 2014, 194, 241–254. [Google Scholar] [CrossRef]
- Moser, L.; Fonti, P.; Buntgen, U.; Esper, J.; Luterbacher, J.; Franzen, J.; Frank, D. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 2010, 30, 225–233. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Čufar, K.; Cuny, H.E.; Deslauriers, A.; Fonti, P.; Frank, D.; Gričar, J.; Gruber, A.; Huang, J.-G.; et al. Pattern of xylem phenology in conifers of cold ecosystems at the Northern Hemisphere. Glob. Chang. Biol. 2016, 22, 3804–3813. [Google Scholar] [CrossRef] [Green Version]
- Hänninen, H. Effects of Climatic Change on Overwintering of Forest Trees in Temperate and Boreal Zones. In Proceedings of the International Conference on Impacts of Global Change on Tree Physiology and Forest Ecosystems, Wageningen, The Netherlands, 26–29 November 1996; Mohren, G.M.J., Kramer, K., Sabaté, S., Eds.; Forestry Sciences. Springer: Dordrecht, The Netherlands, 1997; pp. 149–158, ISBN 978-94-015-8949-9. [Google Scholar]
- Sarvas, R. Investigations on the Annual Cycle of Development of Forest Trees. Active Period; Communicationes Instituti Forestalis Fenniae: Helsinki, Finland, 1972; Volume 76. [Google Scholar]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.T.; Prentice, I.C. Climate change, tree species distributions and forest dynamics: A case study in the mixed conifer/northern hardwoods zone of northern Europe. Clim. Chang. 1996, 34, 161–177. [Google Scholar] [CrossRef]
- Amiro, B.D.; Stocks, B.J.; Alexander, M.E.; Flannigan, M.D.; Wotton, B.M. Fire, climate change, carbon and fuel management in the Canadian boreal forest. Int. J. Wildland Fire 2001, 10, 405–413. [Google Scholar] [CrossRef]
- Walker, X.J.; Baltzer, J.L.; Cumming, S.G.; Day, N.J.; Ebert, C.; Goetz, S.; Johnstone, J.F.; Potter, S.; Rogers, B.M.; Schuur, E.A.G.; et al. Increasing wildfires threaten historic carbon sink of boreal forest soils. Nature 2019, 572, 520–523. [Google Scholar] [CrossRef]
- Gregow, H.; Laaksonen, A.; Alper, M.E. Increasing large scale windstorm damage in Western, Central and Northern European forests, 1951–2010. Sci. Rep. 2017, 7, 46397. [Google Scholar] [CrossRef] [Green Version]
- San Miguel Ayanz, J.; de Rigo, D.; Caudullo, G.; Durrant, T.H.; Mauri, A. European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-36740-3. [Google Scholar]
- Jönsson, A.M.; Linderson, M.-L.; Stjernquist, I.; Schlyter, P.; Bärring, L. Climate change and the effect of temperature backlashes causing frost damage in Picea abies. Glob. Planet. Chang. 2004, 44, 195–207. [Google Scholar] [CrossRef]
- Prentice, I.C.; Sykes, M.T.; Cramer, W. A simulation model for the transient effects of climate change on forest landscapes. Ecol. Model. 1993, 65, 51–70. [Google Scholar] [CrossRef]
- Bradshaw, R.H.; Holmqvist, B.H.; Cowling, S.A.; Sykes, M.T. The effects of climate change on the distribution and management of Picea abies in southern Scandinavia. Can. J. For. Res. 2000, 30, 1992–1998. [Google Scholar] [CrossRef]
- Pitelka, L.; Ash, J.; Berry, S.; Bradshaw, R.; Brubaker, L.B.; Clark, J.; Davis, M.; Dyer, J.; Gardner, R.; Gitay, H.; et al. Plant migration and climate change. Am. Sci. 1997, 85, 464–473. [Google Scholar]
- Schlyter, P.; Stjernquist, I.; Bärring, L.; Jönsson, A.; Nilsson, C. Assessment of the impacts of climate change and weather extremes on boreal forests in northern Europe, focusing on Norway spruce. Clim. Res. 2006, 31, 75–84. [Google Scholar] [CrossRef]
- Jansson, G.; Danusevičius, D.; Grotehusman, H.; Kowalczyk, J.; Krajmerova, D.; Skrøppa, T.; Wolf, H. Norway Spruce (Picea abies (L.) H.Karst.). In Forest Tree Breeding in Europe: Current State-of-the-Art and Perspectives; Pâques, L.E., Ed.; Managing Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2013; pp. 123–176. ISBN 978-94-007-6146-9. [Google Scholar]
- Shingleton, A.W. Allometry: The Study of Biological Scaling. Nat. Educ. Knowl. 2010, 3, 2. [Google Scholar]
- Niklas, K.J. Plant Allometry: The Scaling of Form and Process; University of Chicago Press: Chicago, IL, USA, 1994; ISBN 0-226-58080-6. [Google Scholar]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef] [PubMed]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, L.I.; Dubayah, R.O.; Enquist, B.J. Assessing the general patterns of forest structure: Quantifying tree and forest allometric scaling relationships in the United States: Forest allometric variability in the United States. Glob. Ecol. Biogeogr. 2015, 24, 1465–1475. [Google Scholar] [CrossRef]
- Pilli, R.; Anfodillo, T.; Carrer, M. Towards a functional and simplified allometry for estimating forest biomass. For. Ecol. Manag. 2006, 237, 583–593. [Google Scholar] [CrossRef]
- West, G.B. A General Model for the Origin of Allometric Scaling Laws in Biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef]
- Anfodillo, T.; Petit, G.; Sterck, F.; Lechthaler, S.; Olson, M.E. Allometric Trajectories and “Stress”: A Quantitative Approach. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Enquist, B.J. Universal scaling in tree and vascular plant allometry: Toward a general quantitative theory linking plant form and function from cells to ecosystems. Tree Physiol. 2002, 22, 1045–1064. [Google Scholar] [CrossRef] [Green Version]
- Anfodillo, T.; Carrer, M.; Simini, F.; Popa, I.; Banavar, J.R.; Maritan, A. An allometry-based approach for understanding forest structure, predicting tree-size distribution and assessing the degree of disturbance. Proc. R. Soc. B 2013, 280, 20122375. [Google Scholar] [CrossRef]
- Sellan, G.; Simini, F.; Maritan, A.; Banavar, J.R.; de Haulleville, T.; Bauters, M.; Doucet, J.-L.; Beeckman, H.; Anfodillo, T. Testing a general approach to assess the degree of disturbance in tropical forests. J. Veg. Sci. 2017, 28, 659–668. [Google Scholar] [CrossRef]
- Simini, F.; Anfodillo, T.; Carrer, M.; Banavar, J.R.; Maritan, A. Self-similarity and scaling in forest communities. Proc. Natl. Acad. Sci. USA 2010, 107, 7658–7662. [Google Scholar] [CrossRef] [Green Version]
- Enquist, B.J.; West, G.B.; Brown, J.H. Extensions and evaluations of a general quantitative theory of forest structure and dynamics. Proc. Natl. Acad. Sci. USA 2009, 106, 7046–7051. [Google Scholar] [CrossRef] [Green Version]
- West, G.B.; Enquist, B.J.; Brown, J.H. A general quantitative theory of forest structure and dynamics. Proc. Natl. Acad. Sci. USA 2009, 106, 7040–7045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson-Teixeira, K.J.; McGarvey, J.C.; Muller-Landau, H.C.; Park, J.Y.; Gonzalez-Akre, E.B.; Herrmann, V.; Bennett, A.C.; So, C.V.; Bourg, N.A.; Thompson, J.R.; et al. Size-related scaling of tree form and function in a mixed-age forest. Funct. Ecol. 2015, 29, 1587–1602. [Google Scholar] [CrossRef]
- Muller-Landau, H.C.; Condit, R.S.; Chave, J.; Thomas, S.C.; Bohlman, S.A.; Bunyavejchewin, S.; Davies, S.; Foster, R.; Gunatilleke, S.; Gunatilleke, N.; et al. Testing metabolic ecology theory for allometric scaling of tree size, growth and mortality in tropical forests. Ecol. Lett. 2006, 9, 575–588. [Google Scholar] [CrossRef]
- Russo, S.E.; Wiser, S.K.; Coomes, D.A. Growth-size scaling relationships of woody plant species differ from predictions of the Metabolic Ecology Model. Ecol. Lett. 2007, 10, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Xie, J.-B.; Xu, G.-Q.; Jenerette, G.D.; Bai, Y.; Wang, Z.-Y.; Li, Y. Apparent plasticity in functional traits determining competitive ability and spatial distribution: A case from desert. Sci. Rep. 2015, 5, 12174. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.-L.; Niklas, K.J. Above- and Below-ground Biomass Relationships across 1534 Forested Communities. Ann. Bot. 2007, 99, 95–102. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control: Tansley review. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. USA 2014, 111, 13721–13726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatemi, F.R.; Yanai, R.D.; Hamburg, S.P.; Vadeboncoeur, M.A.; Arthur, M.A.; Briggs, R.D.; Levine, C.R. Allometric equations for young northern hardwoods: The importance of age-specific equations for estimating aboveground biomass. Can. J. For. Res. 2011, 41, 881–891. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A. Allometry and partitioning of above- and belowground tree biomass in an age-sequence of white pine forests. For. Ecol. Manag. 2007, 253, 68–80. [Google Scholar] [CrossRef]
- Poorter, H.; Jagodzinski, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.O.; Lumbres, R.I.C.; Lee, Y.J. Partitioning of above and belowground biomass and allometry in the two stand age classes of Pinus rigida in South Korea. Life Sci. J. 2012, 9, 3553–3559. [Google Scholar]
- Buras, A.; Rammig, A.; Zang, C.S. Quantifying impacts of the 2018 drought on European ecosystems in comparison to 2003. Biogeosciences 2020, 17, 1655–1672. [Google Scholar] [CrossRef] [Green Version]
- Lippestad, H. Cooperation Is a Must for Adaptation to and Mitigation of Climate Change. Available online: https://www.met.no/en/archive/cooperation-is-a-must-for-adaptation-to-and-mitigation-of-climate-change (accessed on 25 May 2020).
- Skogfrøverket Frøplantasje nr. 1122 Opsahl. Available online: http://www.skogfroverket.no/userfiles/files/Fr%C3%B8plantasjeveiledning/Fr%C3%B8kildebeskrivelser_april2018/1122_Opsahl.pdf (accessed on 1 April 2020).
- Skogfrøverket Frøplantasje, nr. 1221 Kaupanger. Available online: http://www.skogfroverket.no/userfiles/files/Fr%C3%B8plantasjeveiledning/Fr%C3%B8kildebeskrivelser_april2018/1221_Kaupanger-Frost.pdf (accessed on 1 April 2020).
- Fløistad, I.S.; Granhus, A. Bud break and spring frost hardiness in Picea abies seedlings in response to photoperiod and temperature treatments. Can. J. For. Res. 2010, 40, 968–976. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Christensen, R.H.B. Ordinal—Regression Models for Ordinal Data. 2019. Available online: https://rdrr.io/cran/ordinal/ (accessed on 17 November 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Barton, K. MuMIn: Multi-Model Inference. 2019. Available online: https://rdrr.io/cran/MuMIn/ (accessed on 17 November 2020).
- Niklas, K.J. Plant allometry: Is there a grand unifying theory? Biol. Rev. 2004, 79, 871–889. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.E.; Hauck, M.; Hajek, P.; et al. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Mäkinen, H.; Nojd, P.; Saranpaa, P. Seasonal changes in stem radius and production of new tracheids in Norway spruce. Tree Physiol. 2003, 23, 959–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Kellomäki, S.; Peltola, H.; Nuutinen, T.; Korhonen, K.T.; Strandman, H. Sensitivity of managed boreal forests in Finland to climate change, with implications for adaptive management. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2339–2349. [Google Scholar] [CrossRef] [Green Version]
- Kauppi, P.E.; Posch, M.; Pirinen, P. Large Impacts of Climatic Warming on Growth of Boreal Forests since 1960. PLoS ONE 2014, 9, e111340. [Google Scholar] [CrossRef] [Green Version]
- Kurz, W.A.; Stinson, G.; Rampley, G. Could increased boreal forest ecosystem productivity offset carbon losses from increased disturbances? Phil. Trans. R. Soc. B 2008, 363, 2259–2268. [Google Scholar] [CrossRef] [Green Version]
- D’Orangeville, L.; Houle, D.; Duchesne, L.; Phillips, R.P.; Bergeron, Y.; Kneeshaw, D. Beneficial effects of climate warming on boreal tree growth may be transitory. Nat. Commun. 2018, 9, 3213. [Google Scholar] [CrossRef] [Green Version]
- Caré, O.; Müller, M.; Vornam, B.; Höltken, A.; Kahlert, K.; Krutovsky, K.; Gailing, O.; Leinemann, L. High Morphological Differentiation in Crown Architecture Contrasts with Low Population Genetic Structure of German Norway Spruce Stands. Forests 2018, 9, 752. [Google Scholar] [CrossRef] [Green Version]
- Geburek, T.; Robitschek, K.; Milasowszky, N. A tree of many faces: Why are there different crown types in Norway spruce (Picea abies [L.] Karst.)? Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 126–133. [Google Scholar] [CrossRef]
- Lines, E.R.; Zavala, M.A.; Purves, D.W.; Coomes, D.A. Predictable changes in aboveground allometry of trees along gradients of temperature, aridity and competition. Glob. Ecol. Biogeogr. 2012, 21, 1017–1028. [Google Scholar] [CrossRef]




| Site | Group | Mean T (°C) |
|---|---|---|
| A (320 m a.s.l.) | b | 17.16 (±0.1) |
| B (420 m a.s.l.) | b | 17.09 (±0.1) |
| C (500 m a.s.l.) | a | 16.39 (±0.1) |
| D (595 m a.s.l.) | a | 16.49 (±0.1) |
| A | B | C | D | |
|---|---|---|---|---|
| A | / | 0.17 (±0.01) | 0.80 (±0.02) | 0.75 (±0.03) |
| B | / | 0.64 (±0.01) | 0.59 (±0.02) | |
| C | / | −0.05 (±0.02) |
| Site | Group | Mean Height (cm) |
|---|---|---|
| A (320 m a.s.l.) | b | 169.97 (±5.2) |
| B (420 m a.s.l.) | b | 166.44 (±5.3) |
| C (500 m a.s.l.) | a | 136.35 (±4.7) |
| D (595 m a.s.l.) | a | 137.47 (±4.7) |
| Site | Group | Mean Diameter (cm) |
| A (320 m a.s.l.) | b | 3.16 (±0.11) |
| B (420 m a.s.l.) | b | 3.19 (±0.10) |
| C (500 m a.s.l.) | a | 2.55 (±0.10) |
| D (595 m a.s.l.) | a | 2.75 (±0.11) |
| Site | Group | Mean Date (DOY) | Approximate Date |
|---|---|---|---|
| A (320 m a.s.l.) | ab | 144.98 (±0.29) | 25 May |
| B (420 m a.s.l.) | a | 144.67 (±0.31) | 25 May |
| C (500 m a.s.l.) | bc | 146.47 (±0.53) | 26 May |
| D (595 m a.s.l.) | c | 146.62 (±0.4) | 27 May |
| Site | Group | Mean Normalized Shoot Elongation | Mean Shoot Elongation (cm) |
|---|---|---|---|
| A | a | 0.22 (±0.01) | 37.48 (±2.2) |
| B | ab | 0.25 (±0.01) | 42.12 (±1.9) |
| C | b | 0.26 (±0.01) | 35.77 (±1.7) |
| D | ab | 0.23 (±0.01) | 32.32 (± 2) |
| Site | Group | Mean Normalized Diameter Increment | Mean Diameter Increment (cm) |
| A | a | 0.105 (±0.007) | 0.334 (±0.02) |
| B | ab | 0.112 (±0.008) | 0.351 (±0.02) |
| C | bc | 0.139 (±0.009) | 0.348 (±0.02) |
| D | c | 0.138 (±0.007) | 0.374 (±0.02) |
| Allometric Relationship | Site | Slope (b) | C.I. (2.5%) | C.I. (97.5%) | Intercept (a) | C.I. (2.5%) | C.I. (97.5%) |
|---|---|---|---|---|---|---|---|
| Diameter Vs. Height (D vs. H) | A | 0.96 | 0.91 | 0.99 | 0.03 | 0.02 | 0.03 |
| B | 0.89 | 0.85 | 0.94 | 0.03 | 0.03 | 0.04 | |
| C | 0.91 | 0.86 | 0.96 | 0.03 | 0.02 | 0.04 | |
| D | 0.93 | 0.88 | 0.98 | 0.03 | 0.02 | 0.04 | |
| Crown Length Vs. Height (Lcro vs. H) | A | 1.12 | 1.1 | 1.15 | 0.48 | 0.43 | 0.54 |
| B | 1.04 | 1.03 | 1.06 | 0.74 | 0.68 | 0.79 | |
| C | 1.04 | 1.02 | 1.06 | 0.71 | 0.66 | 0.79 | |
| D | 1.06 | 1.03 | 1.09 | 0.63 | 0.55 | 0.72 | |
| Crown radius Vs. Height (Rcro vs. H) | A | 0.77 | 0.7 | 0.84 | 1.05 | 0.78 | 1.48 |
| B | 0.84 | 0.78 | 0.91 | 0.69 | 0.5 | 0.95 | |
| C | 0.81 | 0.75 | 0.86 | 0.81 | 0.62 | 1.05 | |
| D | 0.89 | 0.84 | 0.94 | 0.58 | 0.45 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mura, C.; Strømme, C.B.; Anfodillo, T. Stable Allometric Trajectories in Picea abies (L.) Karst. Trees along an Elevational Gradient. Forests 2020, 11, 1231. https://0-doi-org.brum.beds.ac.uk/10.3390/f11111231
Mura C, Strømme CB, Anfodillo T. Stable Allometric Trajectories in Picea abies (L.) Karst. Trees along an Elevational Gradient. Forests. 2020; 11(11):1231. https://0-doi-org.brum.beds.ac.uk/10.3390/f11111231
Chicago/Turabian StyleMura, Claudio, Christian Bianchi Strømme, and Tommaso Anfodillo. 2020. "Stable Allometric Trajectories in Picea abies (L.) Karst. Trees along an Elevational Gradient" Forests 11, no. 11: 1231. https://0-doi-org.brum.beds.ac.uk/10.3390/f11111231