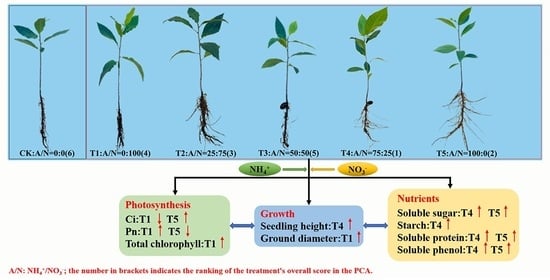
Ammonia–Nitrate Mixture Dominated by NH4+–N Promoted Growth, Photosynthesis and Nutrient Accumulation in Pecan (Carya illinoinensis)
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Experimental Design
2.2. Measurements
2.2.1. Measurement of Growth Parameters
2.2.2. Measurement of Chlorophyll and Photosynthetic Parameters
2.2.3. Measurement of Major Nutrient Elements
2.3. Data Analysis
3. Results
3.1. Effects of N Forms on the Growth Characteristics of Pecan Seedlings
3.2. Effects of N Forms on the Photosynthetic Characteristics of Pecan Seedlings
3.3. Effects of N Forms on the Chlorophyll and Lutein Concentrations of Pecan Seedlings
3.4. Effects of N Forms on the Accumulation of Nutrient Substances in Pecan Seedlings
3.5. Correlation Analysis of Growth Physiological Indexes of Pecan
3.6. Comprehensive Evaluation of Photosynthesis and Growth of Pecan
4. Discussion
4.1. Growth of Pecan Seedlings under Different NH4+:NO3− Ratios
4.2. Photosynthetic Capacity of Pecan Seedlings under Different NH4+:NO3− Ratios
4.3. Nutrient Accumulation in Pecan Seedlings under Different NH4+:NO3− Ratios
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.-X.; Wang, Z.-H.; Stewart, B.A. Responses of Crop Plants to Ammonium and Nitrate N. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 118, pp. 205–397. [Google Scholar]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming ammonium toxicity. Plant Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Asghar, M.A.; Hussain, S.; Bin, C.; Shafiq, I.; Ahmad, I.; Ghafoor, A.; Karim, H.; Iqbal, T.; Yang, W.; et al. Optimal NH4+/NO3− ratios enhance the shade tolerance of soybean seedlings under low light conditions. Plant Biol. 2021, 23, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hasbullah, H.; Setia, R.; Marschner, P.; Zhang, F. Potential soil P mobilisation capacity–method development and comparison of rhizosphere soil from different crops. Plant Soil 2011, 354, 259–267. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Gilhooly, W.P.; Jacinthe, P.-A. Nitrogen preference across generations under changing ammonium nitrate ratios. J. Plant Ecol. 2019, 12, 235–244. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Mokhele, B.; Zhan, X.; Yang, G.; Zhang, X. Review: Nitrogen assimilation in crop plants and its affecting factors. Can. J. Plant Sci. 2012, 92, 399–405. [Google Scholar] [CrossRef]
- Cheng, W.; Sakai, H.; Yagi, K.; Hasegawa, T. Combined effects of elevated CO2 and high night temperature on carbon assimilation, nitrogen absorption, and the allocations of C and N by rice (Oryza sativa L.). Agric. For. Meteorol. 2010, 150, 1174–1181. [Google Scholar] [CrossRef]
- Zhang, H.H.; Liu, H.F.; Li, Z.; Ding, H.F.; Xue, Z.X.; Zhao, F.Y.; Yu, S.L.; Yu, K. Effects of temperature and nitrogen application on photosynthetic characteristics and the absorption and distribution of carbon and nitrogen in apple plants. Photosynthetica 2021, 59, 538–546. [Google Scholar] [CrossRef]
- Urlić, B.; Jukić Špika, M.; Becker, C.; Kläring, H.-P.; Krumbein, A.; Goreta Ban, S.; Schwarz, D. Effect of NO3 and NH4 concentrations in nutrient solution on yield and nitrate concentration in seasonally grown leaf lettuce. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 748–757. [Google Scholar] [CrossRef]
- Ohyama, T. Nitrogen as a Major Essential Element of Plants. In Nitrogen Assimilation in Plants; Takuji, O., Kuni, S., Eds.; Research Signpost: Kerala, India, 2010; pp. 1–18. ISBN 9788130804064. [Google Scholar]
- Quaggio, J.A.; Souza, T.R.; Bachiega Zambrosi, F.C.; Marcelli Boaretto, R.; Mattos, D., Jr. Nitrogen-fertilizer forms affect the nitrogen-use efficiency in fertigated citrus groves. J. Plant Nutr. Soil Sci. 2014, 177, 404–411. [Google Scholar] [CrossRef]
- Epron, D.; Koutika, L.-S.; Tchichelle, S.V.; Bouillet, J.-P.; Mareschal, L. Uptake of soil mineral nitrogen by Acacia mangium and Eucalyptus urophylla × grandis: No difference in N form preference. J. Plant Nutr. Soil Sci. 2016, 179, 726–732. [Google Scholar] [CrossRef]
- Boussadia, O.; Steppe, K.; Zgallai, H.; Ben El Hadj, S.; Braham, M.; Lemeur, R.; Van Labeke, M.C. Effects of nitrogen deficiency on leaf photosynthesis, carbohydrate status and biomass production in two olive cultivars ‘Meski’ and ‘Koroneiki’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.; Laghari, G.; Laghari, A.; Bhabhan, G.; HussainTalpur, K.; Ahmed, T.; Wahocho, S.; Lashari, A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–218. [Google Scholar]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Coskun, D.; Britto, D.T.; Li, M.; Becker, A.; Kronzucker, H.J. Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4+ toxicity in plant roots. Plant Physiol. 2013, 163, 1859–1867. [Google Scholar] [CrossRef] [Green Version]
- Hachiya, T.; Watanabe, C.K.; Fujimoto, M.; Ishikawa, T.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H.; Uesono, Y.; Terashima, I.; Noguchi, K. Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in Arabidopsis thaliana shoots. Plant Cell Physiol. 2012, 53, 577–591. [Google Scholar] [CrossRef]
- Serna, M.D.; Borras, R.; Legaz, F.; Primo-Millo, E. The influence of nitrogen concentration and ammonium/nitrate ratio on N-uptake, mineral composition and yield of citrus. Plant Soil 1992, 147, 13–23. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, B.; Hao, Y.; Liu, H.; Sun, G.; Chen, R.; Song, S. Appropriate NH4+/NO3− ratio triggers plant growth and nutrient uptake of flowering Chinese cabbage by optimizing the pH value of nutrient solution. Front. Plant Sci. 2021, 12, 656144. [Google Scholar] [CrossRef]
- Guo, J.; Jia, Y.; Chen, H.; Zhang, L.; Yang, J.; Zhang, J.; Hu, X.; Ye, X.; Li, Y.; Zhou, Y. Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci. Rep. 2019, 9, 1248. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Zhou, Y.; Shen, Q.; Zhang, F. Effect of ammonium and nitrate nutrition on some physiological processes in higher plants—Growth, photosynthesis, photorespiration, and water relations. Plant Biol. 2007, 9, 21–29. [Google Scholar] [CrossRef]
- Nicodemus, M.A.; Salifu, F.K.; Jacobs, D.F. Growth, nutrition, and photosynthetic response of black walnut to varying nitrogen sources and rates. J. Plant Nutr. 2008, 31, 1917–1936. [Google Scholar] [CrossRef]
- Qin, J.; Yue, X.; Ling, Y.; Zhou, Y.; Li, N.; Shang, X.; Fang, S. Nitrogen form and ratio impact phenolic accumulation and relative gene expression in Cyclocarya paliurus. Trees 2021, 35, 685–696. [Google Scholar] [CrossRef]
- Tabatabaei, S.J.; Yusefi, M.; Hajiloo, J. Effects of shading and NO3:NH4 ratio on the yield, quality and N metabolism in strawberry. Sci. Hortic. 2008, 116, 264–272. [Google Scholar] [CrossRef]
- Mo, Z.; He, H.; Su, W.; Peng, F. Analysis of differentially accumulated proteins associated with graft union formation in pecan (Carya illinoensis). Sci. Hortic. 2017, 224, 126–134. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, F.; Li, Y. Pecan production in China. Sci. Hortic. 2015, 197, 719–727. [Google Scholar] [CrossRef]
- Zhu, K.; Fan, P.; Mo, Z.; Tan, P.; Feng, G.; Li, F.; Peng, F. Identification, eexpression and co-expression analysis of R2R3-MYB family genes involved in graft union formation in pecan (Carya illinoinensis). Forests 2020, 11, 917. [Google Scholar] [CrossRef]
- Smith, M.W.; Wood, B.W.; Raun, W.R. Recovery and partitioning of nitrogen from early spring and midsummer applications to pecan trees. J. Am. Soc. Hortic. Sci. 2007, 132, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Mills, H.A.; Wetzstein, H.Y. Studies on effects of nitrogen form on growth, development, and nutrient uptake in pecan. J. Plant Nutr. 2002, 25, 497–508. [Google Scholar] [CrossRef]
- Liu, G.; Huang, J.; Pan, C.; Wang, Z.; Zheng, B.; Jin, S. Analysis of nitrogen nutrient status of hickory seedlings based on reflection spectroscopy. For. Sci. China 2011, 47, 165–171. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology: Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Rahmani, M.; Atia, G.K. Coherence pursuit: Fast, simple, and robust principal component analysis. IEEE Trans. Signal. Process. 2017, 65, 6260–6275. [Google Scholar] [CrossRef]
- Adams, S.R.; Cockshull, K.E.; Cave, C.R.J. Effect of temperature on the growth and development of tomato fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Roba, T.B. Review on: The effect of mixing organic and inorganic fertilizer on productivity and soil fertility. Open Access Libr. J. 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Wen, Z.; Shen, J.; Blackwell, M.; Li, H.; Zhao, B.; Yuan, H. Combined applications of nitrogen and phosphorus fertilizers with manure increase maize yield and nutrient uptake via stimulating root growth in a long-term experiment. Pedosphere 2016, 26, 62–73. [Google Scholar] [CrossRef]
- Negrini, A.C.A.; Evans, J.R.; Kaiser, B.N.; Millar, A.H.; Kariyawasam, B.C.; Atkin, O.K.; Negrini, A.C.A.; Evans, J.R.; Kaiser, B.N.; Millar, A.H.; et al. Effect of N supply on the carbon economy of barley when accounting for plant size. Funct. Plant Biol. 2020, 47, 368–381. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.; Duan, Z. Effects of elevated CO2 concentration on growth and water usage of tomato seedlings under different ammonium/nitrate ratios. J. Environ. Sci. 2007, 19, 1100–1107. [Google Scholar] [CrossRef]
- Hageman, R.H. Ammonium versus Nitrate Nutrition of Higher Plants. In Nitrogen in Crop Production; Roland, D.H., Ed.; ASA-CSSA-SSSA: Madison, WI, USA, 1984; pp. 67–85. [Google Scholar] [CrossRef]
- Raven, J.A.; Wollenweber, B.; Handley, L.L. A comparison of ammonium and nitrate as nitrogen sources for photolithotrophs. New Phytol. 1992, 121, 19–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.-P.; Cui, J.; Hong, Y.-H.; Luan, Y.-S. Effects of different NH4+/NO3− ratios on the photosynthetic and physiology responses of blueberry (Vaccinium spp.) seedlings growth. J. Plant Nutr. 2021, 44, 854–864. [Google Scholar] [CrossRef]
- Gaastra, P. Photosynthesis of Crop Plants as Influenced by Light, Carbon Dioxide, Temperature, and Stomatal Diffusion Resistance. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 1959. [Google Scholar]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Zhang, T.; Lu, X.; Ellsworth, D.S.; BassiriRad, H.; You, C.; Wang, D.; He, P.; Deng, Q.; Liu, H.; et al. Global response patterns of plant photosynthesis to nitrogen addition: A meta-analysis. Glob. Change Biol. 2020, 26, 3585–3600. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Hui, X.; Wang, Z.; Liu, J. Effects of different fertilization and fallowing practices on soil carbon and nitrogen mineralization in a dryland soil with low organic matter. J. Soil Sci. Plant Nutr. 2019, 19, 108–116. [Google Scholar] [CrossRef]
- Cruz, J.L.; Alves, A.A.C.; LeCain, D.R.; Ellis, D.D.; Morgan, J.A. Effect of elevated CO2 concentration and nitrate: Ammonium ratios on gas exchange and growth of cassava (Manihot esculenta Crantz). Plant Soil 2014, 374, 33–43. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, G.; Liu, H.; Sun, G.; Chen, R.; Song, S. Effects of partial replacement of nitrate with different nitrogen forms on the yield, quality and nitrate content of Chinese kale. Commun. Soil Sci. Plant Anal. 2018, 49, 1384–1393. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars—metabolism, sensing and abiotic stress. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, A.; Deepa, P. Influence of N nutrients on GS activity and putative ammonium transporter1;2 (SaAMT1;2) expression in sandal plants (Santalum album L.). Trees 2017, 31, 1773–1784. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Di Gioia, F.; Kolovou, P.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of Cichorium spinosum L. in relation to nitrate/ammonium nitrogen ratio. J. Sci. Food Agric. 2019, 99, 6741–6750. [Google Scholar] [CrossRef] [Green Version]
- Pfister, B.; Zeeman, S.C. Formation of starch in plant cells. Cell Mol. Life Sci. 2016, 73, 2781–2807. [Google Scholar] [CrossRef] [Green Version]
- Poucet, T.; González-Moro, M.B.; Cabasson, C.; Beauvoit, B.; Gibon, Y.; Dieuaide-Noubhani, M.; Marino, D. Ammonium supply induces differential metabolic adaptive responses in tomato according to leaf phenological stage. J. Exp. Bot. 2021, 72, 3185–3199. [Google Scholar] [CrossRef]
- Deans, C.A.; Behmer, S.T.; Fiene, J.; Sword, G.A. Spatio-temporal, genotypic, and environmental effects on plant soluble protein and digestible carbohydrate content: Implications for insect herbivores with cotton as an exemplar. J. Chem. Ecol. 2016, 42, 1151–1163. [Google Scholar] [CrossRef]
- Xun, Z.; Guo, X.; Li, Y.; Wen, X.; Wang, C.; Wang, Y. Quantitative proteomics analysis of tomato growth inhibition by ammonium nitrogen. Plant Physiol. Biochem. 2020, 154, 129–141. [Google Scholar] [CrossRef]
- Zhu, Z.-B.; Yu, M.-M.; Chen, Y.-H.; Guo, Q.-S.; Zhang, L.-X.; Shi, H.-Z.; Liu, L. Effects of ammonium to nitrate ratio on growth, nitrogen metabolism, photosynthetic efficiency and bioactive phytochemical production of Prunella vulgaris. Pharm. Biol. 2014, 52, 1518–1525. [Google Scholar] [CrossRef] [Green Version]
- Guan, G.-F.; Wang, Y.-S.; Cheng, H.; Jiang, Z.-Y.; Fei, J. Physiological and biochemical response to drought stress in the leaves of Aegiceras corniculatum and Kandelia obovata. Ecotoxicology 2015, 24, 1668–1676. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.; Roberts, T. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]




| Nutrient Source | Treatment | |||||
|---|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | T5 | |
| Ca(H2PO4)2(mM) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| K2SO4(mM) | 1 | 1 | 1 | 1 | 1 | 1 |
| MgSO4(mM) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| ZnSO4(μM) | 1 | 1 | 1 | 1 | 1 | 1 |
| H3BO3(μM) | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| MnSO4(μM) | 1 | 1 | 1 | 1 | 1 | 1 |
| CuSO4(μM) | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| EDTA-Fe(μM) | 10 | 10 | 10 | 10 | 10 | 10 |
| DCD(μM) | 7 | 7 | 7 | 7 | 7 | 7 |
| (Na)6Mo7O24(μM) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| CaCl2(mM) | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 |
| (NH4)2SO4(mM) | 0 | 0 | 0.25 | 0.5 | 0.75 | 1 |
| Ca(NO3)2(mM) | 0 | 1 | 0.75 | 0.5 | 0.25 | 0 |
| Treatment | Ci (ppm) | E (mmol m−2 s−1) | Gs (mmol m−2 s−1) | Pn (μmol m−2 s−1) |
|---|---|---|---|---|
| CK | 453.33 ± 29.95 AB | 0.68 ± 0.13 | 57.25 ± 13.29 | 2.93 ± 1.03 B |
| T1 | 310.00 ± 35.68 B | 0.70 ± 0.13 | 68.00 ± 10.65 | 11.00 ± 1.40 A |
| T2 | 455.50 ± 60.19 AB | 0.68 ± 0.14 | 64.25 ± 12.63 | 5.7 ± 0.84 AB |
| T3 | 409.00 ± 50.42 AB | 0.73 ± 0.08 | 72.00 ± 9.90 | 8.25 ± 2.00 AB |
| T4 | 470.67 ± 62.41 AB | 0.58 ± 0.05 | 54.50 ± 2.53 | 6.57 ± 2.77 AB |
| T5 | 528.00 ± 64.84 A | 0.53 ± 0.08 | 47.75 ± 6.26 | 4.10 ± 1.40 B |
| Seedling Height | Ground Diameter | Pn | Total Chlorophyll | Total Soluble Sugar | Total Starch | Total Soluble Protein | Total Soluble Phenol | |
|---|---|---|---|---|---|---|---|---|
| Ci | 0.041 | 0.173 | −0.727 ** | 0.333 | 0.101 | 0.032 | 0.510 * | 0.331 |
| E | 0.045 | 0.065 | 0.344 | 0.001 | 0.125 | 0.008 | 0.283 | 0.413 |
| Gs | 0.006 | 0.096 | 0.497 * | 0.155 | 0.116 | 0.030 | 0.271 | 0.328 |
| Chlorophyll a | 0.302 | 0.198 | 0.502 | 0.990 ** | 0.279 | 0.203 | 0.109 | 0.023 |
| Chlorophyll b | 0.194 | 0.180 | 0.519 * | 0.986 ** | 0.060 | 0.341 | 0.109 | 0.041 |
| Lutein | 0.278 | 0.197 | 0.518 * | 0.991 ** | 0.223 | 0.246 | 0.050 | 0.008 |
| Leaf Soluble Sugar | 0.231 | 0.073 | 0.047 | −0.514 * | 0.863 ** | 0.334 | 0.390 | 0.308 |
| Stem Soluble Sugar | 0.263 | 0.272 | 0.026 | 0.153 | 0.808 ** | 0.740 ** | 0.748 ** | 0.617 ** |
| Root Soluble Sugar | 0.268 | 0.016 | 0.258 | 0.048 | 0.910 ** | 0.610 ** | 0.501 * | 0.338 |
| Leaf Starch | 0.302 | 0.361 | 0.177 | 0.158 | 0.530 * | 0.994 ** | 0.689 ** | 0.718 ** |
| Stem Starch | 0.505 * | 0.400 | 0.311 | 0.168 | 0.501 * | 0.991 ** | 0.588 ** | 0.694 ** |
| Root Starch | 0.491 * | 0.403 | 0.339 | 0.075 | 0.506 * | 0.993 ** | 0.620 ** | 0.640 ** |
| Leaf Soluble Protein | 0.350 | 0.218 | 0.141 | 0.004 | 0.441 * | 0.566 ** | 0.876 ** | 0.687 ** |
| Stem Soluble Protein | 0.460 * | 0.027 | 0.133 | 0.325 | 0.526 * | 0.509 * | 0.863 ** | 0.487 * |
| Root Soluble Protein | 0.146 | 0.322 | 0.306 | 0.036 | 0.193 | 0.334 | 0.587 ** | 0.644 ** |
| Leaf Soluble Phenol | 0.360 | 0.209 | 0.305 | 0.170 | 0.252 | 0.396 | 0.533 * | 0.822 ** |
| Stem Soluble Phenol | 0.453 * | 0.190 | 0.197 | 0.032 | 0.447 * | 0.520 * | 0.514 * | 0.820 ** |
| Root Soluble Phenol | 0.035 | 0.386 | 0.233 | 0.009 | 0.353 | 0.605 ** | 0.719 ** | 0.756 ** |
| Principal Components | Eigenvalues | Contribution Rate/% | Cumulative Contribution Rate/% |
|---|---|---|---|
| 1 | 6.438 | 42.920 | 42.920 |
| 2 | 5.870 | 39.131 | 82.051 |
| Treatments | Z1 | Z2 | Comprehensive Score | Ranking |
|---|---|---|---|---|
| CK | −0.45 | −3.29 | −1.48 | 6 |
| T1 | −2.76 | 1.50 | −0.60 | 4 |
| T2 | 0.06 | −0.76 | −0.27 | 3 |
| T3 | −2.67 | −0.09 | −1.18 | 5 |
| T4 | 2.57 | 2.34 | 2.02 | 1 |
| T5 | 3.26 | 0.29 | 1.51 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Zhu, K.; Tan, P.; Liu, J.; Xie, J.; Yao, X.; Chu, G.; Peng, F. Ammonia–Nitrate Mixture Dominated by NH4+–N Promoted Growth, Photosynthesis and Nutrient Accumulation in Pecan (Carya illinoinensis). Forests 2021, 12, 1808. https://0-doi-org.brum.beds.ac.uk/10.3390/f12121808
Chen M, Zhu K, Tan P, Liu J, Xie J, Yao X, Chu G, Peng F. Ammonia–Nitrate Mixture Dominated by NH4+–N Promoted Growth, Photosynthesis and Nutrient Accumulation in Pecan (Carya illinoinensis). Forests. 2021; 12(12):1808. https://0-doi-org.brum.beds.ac.uk/10.3390/f12121808
Chicago/Turabian StyleChen, Mengyun, Kaikai Zhu, Pengpeng Tan, Junping Liu, Junyi Xie, Xiuwen Yao, Guolin Chu, and Fangren Peng. 2021. "Ammonia–Nitrate Mixture Dominated by NH4+–N Promoted Growth, Photosynthesis and Nutrient Accumulation in Pecan (Carya illinoinensis)" Forests 12, no. 12: 1808. https://0-doi-org.brum.beds.ac.uk/10.3390/f12121808