Ecological Adaptation of Two Dominant Conifer Species to Extreme Climate in the Tianshan Mountains
Abstract
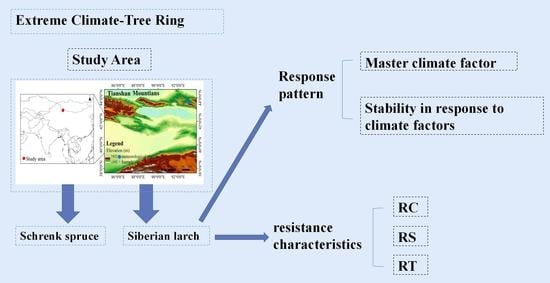
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Tree Ring Sample Collection and Processing
2.3. Chronological Statistical Parameter Calculation
2.4. Meteorological Data and Preprocessing
2.5. Data Analysis
2.5.1. Climate Data Analysis
2.5.2. Analysis of the Relationships between Tree Rings and Climate Factors
2.5.3. Pointer Year Selection
2.5.4. Resistance Index Calculation
3. Results
3.1. Climate Change Characteristics in the Study Area
3.2. Statistical Parameters of Tree-Ring Width Chronologies for the Two Tree Species
3.3. Relationships between Radial Growth and Climatic Factors for the Two Species
3.4. Comparison of the Resistance Indexes of the Two Tree Species to Climate Change
4. Discussion
4.1. Evaluation of the Responses of the Two Tree Species to Extreme Climate
4.2. Comparison of the Resistances of the Two Tree Species to Extreme Climate
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piao, S.; Zhang, X.; Chen, A.; Liu, Q.; Lian, X.; Wang, X.; Peng, S.; Wu, X. The impacts of climate extremes on the terrestrial carbon cycle: A review. Sci. China Earth Sci. 2019, 62, 1551–1563. [Google Scholar] [CrossRef]
- Evans, P.; Brown, C.D. The Boreal-Temperate Forest Ecotone Response to Climate Change. Environ. Rev. 2017, 25, 423–431. [Google Scholar] [CrossRef] [Green Version]
- He, Z.B.; Du, J.; Chen, L.F.; Zhu, X.; Lin, P.F.; Zhao, M.M.; Fang, S. Impacts of recent climate extremes on spring phenology in arid-mountain ecosystems in China. Agric. For. Meteorol. 2018, 260–261, 31–40. [Google Scholar] [CrossRef]
- Siegmund, J.F.; Wiedermann, M.; Donges, J.F.; Donner, R.V. Impact of temperature and precipitation extremes on the flowering dates of four german wildlife shrub species. Biogeosciences 2016, 13, 5541–5555. [Google Scholar] [CrossRef] [Green Version]
- Depardieu, C.; Girardin, M.P.; Nadeau, S.; Lenz, P.; Bousquet, J.; Isabel, N. Adaptive genetic variation to drought in a widely distributed conifer suggests a potential for increasing forest resilience in a drying climate. New Phytol. 2020, 227, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Field, C.B.; Barros, V.; Stocker, T.F.; Dahe, Q. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Suárez-Muñoz, M.; Bonet-García, F.J.; Navarro-Cerrillo, R. Forest management scenarios drive future dynamics of Mediterranean planted pine forests under climate change. Landsc. Ecol. 2023, 1–6. [Google Scholar] [CrossRef]
- Islam, M.; Rahman, M.; Bräuning, A. Impact of extreme drought on tree-ring width and vessel anatomical features of Chukrasia tabularis. Dendrochronologia 2019, 53, 63–72. [Google Scholar] [CrossRef]
- Kramer, P.; Kozlowski, T.T. Physiology of Woody Plants; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Begović, K.; Rydval, M.; Mikac, S.; Čupić, S.; Svobodova, K.; Mikoláš, M.; Kozák, D.; Kameniar, O.; Frankovič, M.; Pavlin, J.; et al. Climate-growth relationships of Norway Spruce and silver fir in primary forests of the Croatian Dinaric mountains. Agric. For. Meteorol. 2020, 12, 288–289. [Google Scholar] [CrossRef]
- Zohner, C.M.; Mo, L.; Sebald, V.; Renner, S.S. Leaf-out in northern ecotypes of wide-ranging trees requires less spring warming, enhancing the risk of spring frost damage at cold range limits. Glob. Ecol. Biogeogr. 2020, 29, 1065–1072. [Google Scholar] [CrossRef]
- Chang, C.T.; Wang, L.J.; Huang, J.C.; Liu, C.P.; Wang, C.P.; Lin, N.H.; Wang, L.X.; Lin, T.C. Precipitation controls on nutrient budgets in subtropical and tropical forests and the implications under changing climate. Adv. Water Resour. 2017, 103, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.C.; Lee, T.Y.; Lin, T.C.; Hein, T.; Lee, L.C.; Shih, Y.T.; Kao, S.J.; Shiah, F.K.; Lin, N.H. Effects of different N sources on riverine DIN export and retention in a subtropical high-standing island Taiwan. Biogeosciences 2016, 13, 1787–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.L.; Fang, Y.C.; Munger, W.; Jiang, P.; Whitby, T.G.; Chen, S.Y.; Wei, W.J.; Xiu, L.M. Precipitation extremes influence patterns and partitioning of evapotranspiration and transpiration in a deciduous boreal larch forest. For. Ecol. Manag. 2020, 287, 107936. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic: London, UK, 1976; p. 582. [Google Scholar]
- Shao, X.M. Progress of tree-ring chronology. Quat. Sci. 1997, 17, 265–271. [Google Scholar]
- Li, C.J.; Chen, T.; Wang, B.; Xu, G.B.; Zhang, X.W.; Wu, G.J. Advances in research on the abnormal structure of tree-rings. Acta Ecol. Sin. 2019, 38, 1538–1550. [Google Scholar]
- Wu, X.D. Tree Rings and Climate Change; Meteorological: Beijing, China, 1990; pp. 65–75. [Google Scholar]
- Gao, S.; Liu, R.S.; Zhou, T.; Fang, W.; Yi, C.X.; Lu, R.J.; Zhao, X.; Luo, H. Dynamic responses of tree-ring growth to multiple dimensions of drought. Glob. Chang. Biol. 2018, 24, 5380–5390. [Google Scholar] [CrossRef] [Green Version]
- Parobeková, Z.; Sedmáková, D.; Kucbel, S.; Pittner, J.; Jaloviar, P.; Saniga, M.; Balanda, M.; Vencurik, J. Influence of disturbances and climate on high-mountain Norway spruce forests in the Low Tatra Mts. Slovakia. For. Ecol Manag. 2016, 380, 128–138. [Google Scholar] [CrossRef]
- DeSoto, L.; Camarero, J.J.; Olano, J.M. Geographically structured and temporally unstable growth responses of Juniperus thurifera to recent climate variability in the Iberian Peninsula. Eur. J. For. Res. 2012, 131, 905–917. [Google Scholar] [CrossRef] [Green Version]
- Hofgaard, A.; Ols, C.; Drobyshev, I.; Kirchhefer, A.J.; Söderström, L. Non-stationary Response of Tree Growth to Climate Trends Along the Arctic Margin. Ecosystems 2019, 22, 434–451. [Google Scholar] [CrossRef] [Green Version]
- Jiao, L.; Chen, K.; Wang, S.J.; Liu, X.P. Stability evaluation of radial growth of Picea schrenkiana in different age groups in response to climate change in the eastern Tianshan Mountains. J. Mt. Sci.-Engl. 2020, 17, 1735–1748. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, D.C.; Tian, K.; Zhang, W.G.; He, R.H.; He, W.Q.; Sun, J.M.; Liu, Z.Y. Radial growth responses of Picea likiangensis to climate variabilities at different altitudes in Yulong Snow Mountain, southwest China. Chin. J. Plant Ecol. 2018, 042, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Altman, J.; Treydte, K.; Pejcha, V.; Cerny, T.; Petrik, P.; Srutek, M.; Song, J.S.; Trouet, V.; Dolezal, J. Tree growth response to recent warming of two endemic species in Northeast Asia. Clim. Chang. 2020, 162, 1345–1364. [Google Scholar] [CrossRef]
- Jia, F.F.; Sun, C.Y.; Sun, H.Y.; Li, X. Responses of radial growth of two dominant tree species to climate change on Changling Mountain. Acta Ecol. Sin. 2019, 39, 6332–6340. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Wang, X.F.; Yang, B.; Li, G. Drought-induced tree growth decline in the desert margins of Northwestern China. Dendrochronologia 2020, 60, 125685. [Google Scholar] [CrossRef]
- Andivia, E.; Ruiz-Benito, P.; Díaz-Martínez, P.; Carro-Martínez, N.; Madrigal-González, J. Inter-specific tolerance to recurrent droughts of pine species revealed in saplings rather than adult trees. For. Ecol. Manag. 2020, 459, 117848. [Google Scholar] [CrossRef]
- Bottero, A.; D'Amato, A.W.; Palik, B.J.; Bradford, J.B.; Fraver, S.; Battaglia, M.A.; Asherin, L.A. Density-dependent vulnerability of forest ecosystems to drought. J. Appl. Ecol. 2017, 54, 1605–1614. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–496. [Google Scholar] [CrossRef]
- Príncipe, A.; van der Maaten, E.; van der Maaten-Theunissen, M.; Struwe, T.; Wilmking, M.; Kreyling, J. Low resistance but high resilience in growth of a major deciduous forest tree (Fagus sylvatica L.) in response to late spring frost in southern Germany. Trees 2017, 31, 743–751. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bottero, A.; Cailleret, M.; Bigler, C.; Fonti, P.; Gessler, A.; Lévesque, M.; Rohner, B.; Weber, P.; Rigling, A.; et al. Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Glob. Chang. Biol. 2019, 25, 3781–3792. [Google Scholar] [CrossRef]
- Cohen, J.; Screen, J.A.; Furtado, J.C.; Barlow, M.; Whittleston, D.; Coumou, D.; Francis, J.; Dethloff, K.; Entekhabi, D.; Overland, J.; et al. Recent arctic amplification and extreme mid-latitude weather. Nat. Geosci. 2014, 7, 627–637. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University: Cambridge, UK, 2013. [Google Scholar]
- Qin, L.; Yuan, Y.J.; Shang, H.M.; Yu, S.L.; Liu, W.P.; Zhang, R.B. Impacts of Global Warming on the Radial Growth and Long-Term Intrinsic Water-Use Efficiency (iWUE) of Schrenk Spruce (Picea schrenkiana Fisch. et Mey) in the Sayram Lake Basin, Northwest China. Forests 2020, 11, 380. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.B. Responses of the upper and lower tree-ring width of the Schrenk spruce forest in the central Tianshan Mountains to climate change. Chin. J. Appl. Environ. Biol. 2017, 5, 1–12. (In Chinese) [Google Scholar]
- Zhang, R.B.; Yuan, Y.J.; Gou, X.H.; Qing, H. Tree-ring-based moisture variability in western Tianshan Mountains since AD 1882 and its possible driving mechanism. Agric. For. Meteorol. 2016, 218, 267–276. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–95. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree Ring Standardization. Doctoral Dissertation, University of Arizona, Tucson, AZ, USA, 1985. [Google Scholar]
- Ali, S.; Eum, H.I.; Cho, J.; Li, D.; Khan, F.; Firdos, K.; Dairaku, K.; Lall, S.M.; Syewoon, H.; Wajid, N.; et al. Assessment of climate extremes in future projections downscaled by multiple statistical downscaling methods over Pakistan. Atmos. Res. 2019, 222, 114–133. [Google Scholar] [CrossRef]
- Bonsal, B.R.; Zhang, X.; Vincent, L.A.; Hogg, W.D. Characteristics of daily and extreme temperatures over Canada. J. Clim. 2001, 14, 1959–1976. [Google Scholar] [CrossRef]
- Zhao, N.; Jiao, Y.M.; Ma, T.; Zhao, M.M.; Yue, T.X. Estimating the effect of urbanization on extreme climate events in the Beijing-Tianjin-Hebei region, China. Sci. Total Environ. 2019, 688, 1005–1015. [Google Scholar] [CrossRef]
- Pallardy, S.G. Physiology of Woody Plants; Academic: London, UK, 2010. [Google Scholar]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [Green Version]
- Will, R.E.; Wilson, S.M.; Zou, C.B.; Hennessey, H.C. Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest-grassland ecotone. New Phytol. 2013, 200, 366–374. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why dosome plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, J.L.; Wei, H.X. The occurrence of “back spring cold” weather and its influence on the spring growth of trees in China. North. Hortic. 2015, 16, 195–201. [Google Scholar] [CrossRef]
- Wang, X.C.; Pederson, N.; Chen, Z.J.; Lawton, K.; Zhu, C.; Han, S.J. Recent rising temperatures drive younger and southern Korean pine growth decline. Sci. Total Environ. 2019, 649, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Abaimov, A.P. Geographical distribution and genetics of Siberian larch species. In Permafrost Ecosystems: Siberian Larch Forests; Osawa, A., Zyryanova, O.A., Matsuura, Y., Kajimoto, T., Wein, R.W., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 41–58. [Google Scholar]
- Peng, J.F.; Gou, X.H.; Chen, F.H.; Li, J.B. Climatic records of tree-ring width in Picea schrenkiana Fisch and Larix sibirica Ledb. Ecol. Environ. 2005, 14, 460–465. (In Chinese) [Google Scholar]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 1992, 62, 365–392. [Google Scholar] [CrossRef]
- King, G.; Fonti, P.; Nievergelt, D.; Buentgen, U.; Frank, D. Climatic drivers of hourly to yearly tree radius variations along a 6 degrees C natural warming gradient. Agric. For. Meteorol. 2013, 168, 36–46. [Google Scholar] [CrossRef]
- Mooney, H.A.; Dunn, E.L. Convergent evolution of Mediterranean-climate evergreen sclerophyll shrubs. Evolution 1970, 24, 292–303. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef] [Green Version]
- Schneider, E.E.; Affleck, D.L.R.; Larson, A.J. Tree spatial patterns modulate peak snow accumulation and snow disappearance. For. Ecol. Manag. 2019, 441, 9–19. [Google Scholar] [CrossRef]
- Storck, P.; Lettenmaier, D.P.; Bolton, S.M. Measurement of snow interception and canopy effects on snow accumulation and melt in a mountainous maritime climate, Oregon, United States. Water Resour. Res. 2002, 38, 5-1–5-16. [Google Scholar] [CrossRef] [Green Version]
- Watson, E.; Luckman, B.H. An investigation of the snowpack signal in moisture-sensitive trees from the Southern Canadian Cordillera. Dendrochronologia 2016, 38, 118–130. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, K.W.; Lourens, P.; Frans, B.; Borghetti, F.; Jacobs, L.; van Langevelde, F. Relative growth rate variation of evergreen and deciduous savanna tree species is driven by different traits. Ann. Bot.-Lond. 2014, 114, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, K.W.; van Langevelde, F.; Ward, D.; Bongers, F.; da Silva, D.A.; Prins, H.H.T.; de Bie, S.; Sterck, F.J. Deciduous and evergreen trees differ in juvenile biomass allometries because of differences in allocation to root storage. Ann. Bot.-Lond. 2013, 112, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Castellano, P.L.; Srur, A.M.; Lucas, O.; Bianchi, L.O. Climate-growth relationships of deciduous and evergreen Nothofagus species in Southern Patagonia, Argentina. Dendrochronologia 2019, 58, 125646. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; López, B.C.; Loepfe, L.; Lloret, F. Stand-and tree-level determinants of the drought response of Scots pine radial growth. Oecologia 2012, 168, 877–888. [Google Scholar] [CrossRef]
- Zhou, B.; Fan, Z.X.; Qi, J.H. Inter-annual radial growth of evergreen and deciduous tree species and their response to climatic factors in a montane moist evergreen broad-leaved forest in the Ailao Mountains, Southwest China. Acta Ecol. Sincia 2020, 40, 1699–1708. [Google Scholar] [CrossRef]
- Wen, K.G.; Shi, Y.G. China Meteorological Disaster Dictionary, Xinjiang Volume; Meteorological: Beijing, China, 2006. [Google Scholar]
- Liu, B. The weather process in northern Xinjiang is frequent, and the low temperature repeatedly breaks the extreme value; the precipitation in southern Xinjiang is unevenly distributed and the temperature is close to normal. Desert Oasis Meteorol. 2003, 26, 33–34. (In Chinese) [Google Scholar]
- Vaganov, E.A.; Hughes, M.K.; Kirdyanov, A.V.; Schweingruber, F.H.; Silkin, P.P. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 1999, 400, 149–151. [Google Scholar] [CrossRef]
- Egger, B.; Einig, W.; Sclereth, A.; Wallenda, T.; Magel, E.; Loewe, A.; Hampp, R. Carbohydrate metabolism in one- and two-year-old spruce needles, and stem carbohydrates from three months before until three months after bud break. Physiol. Plant. 1996, 96, 91–100. [Google Scholar] [CrossRef]
- Vitasse, Y.; Schneider, L.; Rixen, C.; Christen, D.; Rebetez, M. Increase in the risk of exposure of forest and fruit trees to spring frosts at higher elevations in Switzerland over the last four decades. Agric. For. Meteorol. 2018, 248, 60–69. [Google Scholar] [CrossRef]
- Taschler, D.; Neuner, G. Summer frost resistance and freezing patterns measured in situ in leaves of major alpine plant growth forms in relation to their upper distribution boundary. Plant Cell Environ. 2004, 27, 737–746. [Google Scholar] [CrossRef]
- Bigler, C.; Bugmann, H. Climate-induced shifts in leaf unfolding and frost risk of European trees and shrubs. Sci. Rep. 2018, 8, 9865. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2020, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Poulter, B.; Pederson, N.; Liu, H.; Zhu, Z.; D’Arrigo, R.; Ciais, P.; Davi, N.; Frank, D.; Leland, D.; Myneni, R.; et al. Recent trends in Inner Asian forest dynamics to temperature and precipitation indicate high sensitivity to climate change. Agric. For. Meteorol. 2013, 178–179, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Jiao, L.; Jiang, Y.J.; Zhang, W.T.; Wang, M.C.; Wang, S.J.; Liu, X.R. Assessing the stability of radial growth responses to climate change by two dominant conifer trees species in the tianshan mountains, northwest China. For. Ecol. Manag. 2019, 466, 667–677. [Google Scholar] [CrossRef]
- Sillmann, J.; Roeckner, E. Indices for extreme events in projections of anthropogenic climate change. Clim. Chang. 2008, 86, 83–104. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.Y.; Liu, H.Y.; Anenkhonov, O.A.; Korolyuk, A.Y.; Sandanov, D.V.; Balsanova, L.D.; Naidanov, B.B.; Wu, X.C. Long-term forest resilience to climate change indicated by mortality, regeneration, and growth in semiarid southern Siberia. Glob. Chang. Biol. 2016, 23, 2370–2382. [Google Scholar] [CrossRef] [PubMed]







| Species | Schrenk Spruce | Siberian Larch |
|---|---|---|
| Altitude (m) | 2552 | 2590 |
| Latitude Longitude | 43°32.100′ N | 43°32.085′ N |
| 92°56.329′ E | 92°56.662′ E | |
| Slope | 27° | 33° |
| Aspect | Northeast (40° from North) | North |
| Canopy closure | 0.3 | 0.2 |
| Average tree spacing (m) | 3 | 4.5 |
| Average breast diameter (cm) | 32.4 | 33.8 |
| Average Tree height (m) | 12.3 | 9.7 |
| Average Crown width (m) | 2.7 | 3.7 |
| Statistical Parameters | Schrenk Spruce | Siberian Larch |
|---|---|---|
| Sample depth (core/tree number) | 60/30 | 58/29 |
| Sequence length | 1735–2012 (288) | 1761–2012 (252) |
| Standard deviation (SD) | 0.313 | 0.312 |
| Mean sensitivity (MS) | 0.196 | 0.214 |
| AC1 | 0.681 | 0.712 |
| Correlation coefficient ® | 0.483 | 0.382 |
| Mean correlation among trees (R1) | 0.771 | 0.723 |
| Mean correlation between trees (R2) | 0.475 | 0.366 |
| First principal comment (PC1) | 0.507 | 0.435 |
| Signal-to-noise ratio (SNR) | 36.366 | 14.213 |
| Expressed population signal (EPS) | 0.973 | 0.934 |
| Type | Class | ID | Indicator Name | Definitions | Unit |
|---|---|---|---|---|---|
| Extreme temperature indexes | Extreme temperature warm indexes | TXx | Max Tmax | Monthly maximum value of daily maximum temp | °C |
| TXn | Min Tmax | Monthly minimum value of daily maximum temp | °C | ||
| TX90p | Warm days | Percentage of days when TX > 90th percentile | d | ||
| TN90p | Warm nights | Percentage of days when TN > 90th percentile | d | ||
| WSDI | Warm spell duration indicator | Annual count of days with at least 6 consecutive days when TX > 90th percentile | d | ||
| SU25 | Summer days | Annual count when TX (daily maximum) > 25 °C | d | ||
| TR20 | Tropical nights | Annual count when TN (daily minimum) > 20 °C | d | ||
| Extreme temperature cold indexes | TNx | Max Tmin | Monthly maximum value of daily minimum temp | °C | |
| TNn | Min Tmin | Monthly minimum value of daily minimum temp | °C | ||
| TX10p | Cool days | Percentage of days when TX < 10th percentile | d | ||
| TN10p | Cool nights | Percentage of days when TN < 10th percentile | d | ||
| CSDI | Cold spell duration indicator | Annual count of days with at least 6 consecutive days when TN < 10th percentile | d | ||
| FD0 | Frost days | Annual count when TN (daily minimum) < 0 °C | d | ||
| ID0 | Ice days | Annual count when TX (daily maximum) < 0 °C | d | ||
| Other temperature indexes | GSL | Growing season length | Annual (1st Jan to 31st Dec in NH, 1st July to 30th June in SH) count between first span of at least 6 days with TG > 5 °C and first span after July 1 (January 1 in SH) of 6 days with TG < 5 °C | d | |
| DTR | Diurnal temperature range | Monthly mean difference between TX and TN | °C | ||
| Extreme precipitation indexes | Precipitation frequency indexes | R10 | Number of heavy precipitation days | Annual count of days when PRCP ≥ 10 mm | d |
| R20 | Number of very heavy precipitation days | Annual count of days when PRCP ≥ 20 mm | d | ||
| Rnn | Number of days above nn mm | Annual count of days when PRCP ≥ nn mm, nn is user defined threshold | d | ||
| CDD | Consecutive dry days | Maximum number of consecutive days with RR < 1 mm | d | ||
| CWD | Consecutive wet days | Maximum number of consecutive days with RR ≥ 1 mm | d | ||
| Precipitation magnitude indexes | RX1day | Max 1-day precipitation amount | Monthly maximum 1-day precipitation | mm | |
| Rx5day | Max 5-day precipitation amount | Monthly maximum consecutive 5-day precipitation | mm | ||
| R95p | Very wet days | Annual total PRCP when RR > 95th percentile | mm | ||
| R99p | Extremely wet days | Annual total PRCP when RR > 99th percentile | mm | ||
| PRCPtot | Annual total wet-day precipitation | Annual total PRCP in wet days (RR ≥ 1 mm) | mm | ||
| Precipitation intensity index | SDII | Simple daily intensity index | Annual total precipitation divided by the number of wet days (defined as PRCP ≥ 1.0 mm) in the year | mm/ day |
| ID | Unary Linear Regression Equation | R2 | p Value of Equation | Mutation Year | p Value of Pettitt Test |
|---|---|---|---|---|---|
| Temp | y = 0.3100 + 0.0611x | 0.6200 | 1.3 × 10−12 ** | 1985 | 9.3 × 10−9 ** |
| TXx | y = 30.700 + 0.0308x | 0.1100 | 0.011 * | 1996 | 0.01202 * |
| TXn | y = −22.100 + 0.0707x | 0.0940 | 0.023 * | 1979 | 0.0843 |
| TX90p | y = 6.5800 + 0.11x | 0.1900 | 0.001 ** | 1995 | 0.0004654 ** |
| TN90p | y = −1.3800 + 0.382x | 0.6900 | 2.9 × 10−15 ** | 1984 | 4.415 × 10−9 ** |
| WSDI | y = 0.5050 + 0.127x | 0.1200 | 0.0085 ** | 1995 | 0.03846 * |
| SU25 | y = 35.600 + 0.412x | 0.2600 | 6.9 × 10−5 ** | 1995 | 4.995 × 10−5 ** |
| TR20 | y = −0.0034 + 0.0032x | 0.0490 | 0.1000 | 1987 | 1.501 |
| TNx | y = 13.6000 + 0.1060x | 0.5400 | 1.6 × 10−10 ** | 1984 | 3.522 × 10−8 ** |
| TNn | y = −38.6000 + 0.1450x | 0.3200 | 5.5 × 10−6 ** | 1984 | 1.931 × 10−5 ** |
| TX10p | y = 13.50–0 − 0.1170x | 0.2800 | 3.2 × 10−5 ** | 1993 | 0.0007737 ** |
| TN10p | y = 21.20–0 − 0.3750x | 0.7000 | 2.4 × 10−15 ** | 1985 | 4.002 × 10−9 ** |
| CSDI | y = 10.40–0 − 0.2040x | 0.1700 | 0.0016 ** | 1988 | 0.002104 ** |
| FD0 | y = 2–6 − 0.7110x | 0.7200 | 3.4 × 10−16 ** | 1985 | 7.16 × 10−9 ** |
| ID0 | y = 1–8 − 0.2310x | 0.0770 | 0.0400 * | 1985 | 0.03497 * |
| GSL | y = 175 + 0.2910x | 0.1400 | 0.005 ** | 1994 | 0.02881 * |
| DTR | y = 16.70–0 − 0.0662x | 0.7500 | 1 × 10−17 ** | 1984 | 3.232 × 10−9 ** |
| R10 | y = 3.8900 + 0.0597x | 0.1700 | 0.0016 ** | 1982 | 0.006723 ** |
| R20 | y = 0.7330 + 0.0212x | 0.1000 | 0.016 * | 1986 | 0.05441 |
| Rnn | y = 0.3660 + 0.0071x | 0.0320 | 0.1900 | 1985 | 0.3658 |
| CDD | y = 53.7000 + 0.0521x | 0.0013 | 0.7900 | 1981 | 0.4919 |
| CWD | y = 3.0100 + 0.00491x | 0.0066 | 0.5600 | 1966 | 0.6511 |
| RX1day | y = 22.5000 + 0.1420x | 0.0560 | 0.0820 | 1985 | 0.06505 |
| RX5day | y = 31.8000 + 0.1710x | 0.0420 | 0.1300 | 1982 | 0.1928 |
| R95p | y = 24 + 0.769x | 0.1200 | 0.0080 ** | 1986 | 0.0333 * |
| R99p | y = 1.8800 + 0.4070x | 0.0960 | 0.0210 * | 1983 | 0.1893 |
| PRCPtot | y = 194 + 0.9600x | 0.1200 | 0.0100 * | 1983 | 0.07576 |
| SDII | y = 4.6100 + 0.0279x | 0.2000 | 0.0006 ** | 1981 | 0.001395 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Jiao, L.; Liu, X.; Xue, R.; Qi, C.; Du, D. Ecological Adaptation of Two Dominant Conifer Species to Extreme Climate in the Tianshan Mountains. Forests 2023, 14, 1434. https://0-doi-org.brum.beds.ac.uk/10.3390/f14071434
Wu X, Jiao L, Liu X, Xue R, Qi C, Du D. Ecological Adaptation of Two Dominant Conifer Species to Extreme Climate in the Tianshan Mountains. Forests. 2023; 14(7):1434. https://0-doi-org.brum.beds.ac.uk/10.3390/f14071434
Chicago/Turabian StyleWu, Xuan, Liang Jiao, Xiaoping Liu, Ruhong Xue, Changliang Qi, and Dashi Du. 2023. "Ecological Adaptation of Two Dominant Conifer Species to Extreme Climate in the Tianshan Mountains" Forests 14, no. 7: 1434. https://0-doi-org.brum.beds.ac.uk/10.3390/f14071434