Radix Glycyrrhizae Preparata Induces Cell Cycle Arrest and Induced Caspase-Dependent Apoptosis in Glioblastoma Multiforme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Cell Culture
2.3. Cell Viability in GBM 8401 Cell and U87MG Cell after RGP Treatment
2.4. The Synergistic Therapeutic Effect of RGP and Radiation
2.5. Cell Cycle Analysis
2.6. Assessment of Apoptosis
2.7. Caspase3 Activity Assay
2.8. Migration Assay
2.9. Invasion Assay
2.10. Adhesion Assay
2.11. Western Blotting
2.12. Mitotic Index Analysis
2.13. Data Analysis
3. Result
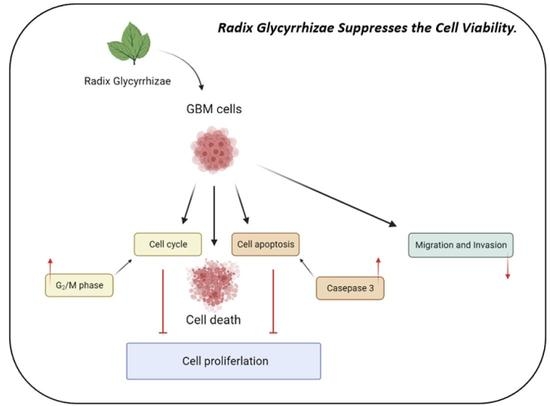
3.1. RGP Suppressed the Cell Viability of GBM8401 and U87MG Cells
3.2. RGP Induced the Caspase-Dependent Apoptosis of GBM8401 Cells
3.3. RGP Attenuates the Migration, Invasion and Adhesion of Glioma Cells
3.4. RGP Triggers G2/M Cell Cycle Arrest in U87MG and GBM8401 Cells
3.5. RGP Modulated the Expression of Cell Cycle-Related Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (Apaf-1) | Apoptotic protease activating factor 1 |
| (Bax) | BCL2 Associated X |
| (Bcl-2) | B-cell lymphoma 2 |
| (CDK2) | Cyclin-dependent kinase 2 |
| (CDK4) | Cyclin-dependent kinase 4 |
| (CHK1) | Checkpoint Kinase 1 |
| (CHK2) | Checkpoint Kinase 2 |
| (ERK) | Extracellular signal-regulated kinase |
| (FADD) | Fas-associated protein with death domain |
| (GBM) | Glioblastoma multiforme |
| (GA) | Glycyrrhizic acid |
| (HMGB1) | High Mobility Group Protein 1 |
| (IAA) | Isoangustone A |
| (ILQ) | Iso-Liquiritigenin |
| (LicA) | Licochalcone A |
| (LQ) | Liquiritin |
| (MG) | Malignant Glioma |
| (MAPK) | Mitogen-activated protein kinase |
| (NF-κB) | Nuclear factor-κB |
| (PI3K) | Phosphatidylinositol 3-kinase |
| (PARP) | Poly ADP-ribose polymerase |
| (RG) | Radix Glycyrrhizae |
| (RGP) | Radix Glycyrrhizae Preparata |
| (Rb) | Retinoblastoma protein |
| (WHO) | World Health Organization |
References
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glas, M.; Happold, C.; Rieger, J.; Wiewrodt, D.; Bähr, O.; Steinbach, J.P.; Wick, W.; Kortmann, R.D.; Reifenberger, G.; Weller, M.; et al. Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J. Clin. Oncol. 2009, 27, 1257–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, J.; Butowski, N.; Chang, S. Recent advances in therapy for glioblastoma. Arch. Neurol. 2010, 67, 279–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gilbert, M.R.; Chakravarti, A. Chemoradiotherapy in malignant glioma: Standard of care and future directions. J. Clin. Oncol. 2007, 25, 4127–4136. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Vijayaraghavan, P.; Chiang, W.H.; Chen, H.H.; Liu, T.I.; Shen, M.Y.; Omoto, A.; Kamimura, M.; Soga, K.; Chiu, H.C. Targeted Delivery of Functionalized Upconversion Nanoparticles for Externally Triggered Photothermal/Photodynamic Therapies of Brain Glioblastoma. Theranostics 2018, 8, 1435–1448. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Z.; Liu, R.; Wu, Y.; Zhang, X. Combined-therapeutic strategies synergistically potentiate glioblastoma multiforme treatment via nanotechnology. Theranostics 2020, 10, 3223–3239. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Yokoshima, K.; Chiba, R.; Fujii, I.; Fattokhov, I.; Saidov, M. Field Survey of Glycyrrhiza Plants in Central Asia (5). Chemical Characterization of G. bucharica Collected in Tajikistan. Chem. Pharm. Bull. 2019, 67, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Sung, M.W.; Li, P.C. Chemical analysis of raw, dry-roasted, and honey-roasted licorice by capillary electrophoresis. Electrophoresis 2004, 25, 3434–3440. [Google Scholar] [CrossRef] [PubMed]
- Majima, T.; Yamada, T.; Tega, E.; Sakurai, H.; Saiki, I.; Tani, T. Pharmaceutical evaluation of liquorice before and after roasting in mice. J. Pharm. Pharmacol. 2004, 56, 589–595. [Google Scholar] [CrossRef]
- Hwang, I.K.; Lim, S.S.; Choi, K.H.; Yoo, K.Y.; Shin, H.K.; Kim, E.J.; Yoon-Park, J.H.; Kang, T.C.; Kim, Y.S.; Kwon, D.Y.; et al. Neuroprotective effects of roasted licorice, not raw form, on neuronal injury in gerbil hippocampus after transient forebrain ischemia. Acta Pharmacol. Sin. 2006, 27, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lim, S.S.; Jung, J.Y.; Choi, J.S.; Kim, J.K.; Han, S.J.; Kang, Y.H. Blockade of nitroxidative stress by roasted licorice extracts in high glucose-exposed endothelial cells. J. Cardiovasc. Pharmacol. 2008, 52, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Jeong, C.K.; Park, K.K.; Choi, J.H.; Park, J.H.; Lim, S.S.; Chung, W.Y. Anti-inflammatory effects of licorice and roasted licorice extracts on TPA-induced acute inflammation and collagen-induced arthritis in mice. J. Biomed. Biotechnol. 2010, 2010, 709378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Wu, L.; Hu, M.; Dong, W.; Xu, M.; Zhang, P. Glycyrrhizic acid: A promising carrier material for anticancer therapy. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 95, 670–678. [Google Scholar] [CrossRef]
- Wang, J.R.; Li, T.Z.; Wang, C.; Li, S.M.; Luo, Y.H.; Piao, X.J.; Feng, Y.C.; Zhang, Y.; Xu, W.T.; Zhang, Y.; et al. Liquiritin inhibits proliferation and induces apoptosis in HepG2 hepatocellular carcinoma cells via the ROS-mediated MAPK/AKT/NF-κB signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, W.; Chen, Y.; Liu, X.; Wang, J.; Qin, X.; Yuan, D.; Yu, T.; Chen, G.; Mi, Y.; et al. Glycyrrhizin Suppresses the Growth of Human NSCLC Cell Line HCC827 by Downregulating HMGB1 Level. BioMed Res. Int. 2018, 2018, 6916797. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, M.; Toyohara, M.; Ueda, S.; Shiroi, A.; Takeuchi, H.; Nishiyama, T.; Yamada, T.; Fukui, H.; Ishizaka, S. Glycyrrhizin inhibits TNF-induced, but not Fas-mediated, apoptosis in the human hepatoblastoma line HepG2. Biol. Pharm. Bull. 1999, 22, 951–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yang, H.; Yue, S.; He, G.; Qu, S.; Zhang, Z.; Ma, B.; Ding, R.; Peng, W.; Zhang, H.; et al. The mTOR inhibition in concurrence with ERK1/2 activation is involved in excessive autophagy induced by glycyrrhizin in hepatocellular carcinoma. Cancer Med. 2017, 6, 1941–1951. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.C.; Chu, P.Y.; Liao, W.T.; Wu, M.Y.; Tsui, K.H.; Lin, L.T.; Huang, C.H.; Chen, L.L.; Li, C.J. Glycyrrhizic acid induces human MDA-MB-231 breast cancer cell death and autophagy via the ROS-mitochondrial pathway. Oncol. Rep. 2018, 39, 703–710. [Google Scholar] [CrossRef]
- Farooqui, A.; Khan, F.; Khan, I.; Ansari, I.A. Glycyrrhizin induces reactive oxygen species-dependent apoptosis and cell cycle arrest at G(0)/G(1) in HPV18(+) human cervical cancer HeLa cell line. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 97, 752–764. [Google Scholar] [CrossRef]
- Thirugnanam, S.; Xu, L.; Ramaswamy, K.; Gnanasekar, M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol. Rep. 2008, 20, 1387–1392. [Google Scholar] [PubMed]
- He, S.Q.; Gao, M.; Fu, Y.F.; Zhang, Y.N. Glycyrrhizic acid inhibits leukemia cell growth and migration via blocking AKT/mTOR/STAT3 signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 5175–5181. [Google Scholar] [PubMed]
- Afnan, Q.; Kaiser, P.J.; Rafiq, R.A.; Nazir, L.A.; Bhushan, S.; Bhardwaj, S.C.; Sandhir, R.; Tasduq, S.A. Glycyrrhizic acid prevents ultraviolet-B-induced photodamage: A role for mitogen-activated protein kinases, nuclear factor kappa B and mitochondrial apoptotic pathway. Exp. Dermatol. 2016, 25, 440–446. [Google Scholar] [CrossRef]
- Wang, Y.M.; Du, G.Q. Glycyrrhizic acid prevents enteritis through reduction of NF-κB p65 and p38MAPK expression in rat. Mol. Med. Rep. 2016, 13, 3639–3646. [Google Scholar] [CrossRef] [Green Version]
- Gong, G.; Xiang, L.; Yuan, L.; Hu, L.; Wu, W.; Cai, L.; Yin, L.; Dong, H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS ONE 2014, 9, e89450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.H.; Liu, H.G.; Zhou, Y.F.; Yue, Q.F. Liquiritin (LT) exhibits suppressive effects against the growth of human cervical cancer cells through activating Caspase-3 in vitro and xenograft mice in vivo. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 92, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Jiang, X.; Gao, H.Y.; Gao, S.H. Liquiritin induces apoptosis and autophagy in cisplatin (DDP)-resistant gastric cancer cells in vitro and xenograft nude mice in vivo. Int. J. Oncol. 2017, 51, 1383–1394. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.S.; Wen, S.H. Effect of early use of Chinese herbal products on mortality rate in patients with lung cancer. J. Ethnopharmacol. 2018, 211, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Casciola-Rosen, L.; Rosen, A.; Petri, M.; Schlissel, M. Surface blebs on apoptotic cells are sites of enhanced procoagulant activity: Implications for coagulation events and antigenic spread in systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 1996, 93, 1624–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Engeland, M.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996, 24, 131–139. [Google Scholar] [CrossRef]
- Chen, P.C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc. Natl. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Shu, J.; He, C.; Li, M.; Wang, Y.; Ou, W.; He, Y. ROCK inhibitor Y27632 promotes proliferation and diminishes apoptosis of marmoset induced pluripotent stem cells by suppressing expression and activity of caspase 3. Theriogenology 2016, 85, 302–314. [Google Scholar] [CrossRef]
- Zheng, W.H.; Quirion, R. Glutamate acting on N-methyl-D-aspartate receptors attenuates insulin-like growth factor-1 receptor tyrosine phosphorylation and its survival signaling properties in rat hippocampal neurons. J. Biol. Chem. 2009, 284, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Goto, H.; Tomono, Y.; Ajiro, K.; Kosako, H.; Fujita, M.; Sakurai, M.; Okawa, K.; Iwamatsu, A.; Okigaki, T.; Takahashi, T.; et al. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J. Biol. Chem. 1999, 274, 25543–25549. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef] [Green Version]
- Stergiou, L.; Hengartner, M.O. Death and more: DNA damage response pathways in the nematode C. elegans. Cell Death Differ. 2004, 11, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Sankari, S.L.; Masthan, K.M.; Babu, N.A.; Bhattacharjee, T.; Elumalai, M. Apoptosis in cancer—An update. Asian Pac. J. Cancer Prev. 2012, 13, 4873–4878. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, D.; Chen, W.; Yan, Z.; Shi, Y. Proteolytic processing of the caspase-9 zymogen is required for apoptosome-mediated activation of caspase-9. J. Biol. Chem. 2013, 288, 15142–15147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, E.H.; Hong, H.D.; Ahn, N.C.; Jung, J.W.; Yang, S.R.; Park, J.S.; Kim, S.H.; Lee, Y.S.; Kang, K.S. Modulations of the Bcl-2/Bax family were involved in the chemopreventive effects of licorice root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast cancer cell. J. Agric. Food Chem. 2004, 52, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.H.; Kim, S.H.; Ra, J.C.; Kim, S.R.; Cho, S.D.; Jung, J.W.; Yang, S.R.; Park, J.S.; Hwang, J.W.; Aruoma, O.I.; et al. Chemopreventive properties of the ethanol extract of chinese licorice (Glycyrrhiza uralensis) root: Induction of apoptosis and G1 cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett. 2005, 230, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Seon, M.R.; Park, S.Y.; Kwon, S.J.; Lim, S.S.; Choi, H.J.; Park, H.; Lim, D.Y.; Kim, J.S.; Lee, C.H.; Kim, J.; et al. Hexane/ethanol extract of Glycyrrhiza uralensis and its active compound isoangustone A induce G1 cycle arrest in DU145 human prostate and 4T1 murine mammary cancer cells. J. Nutr. Biochem. 2012, 23, 85–92. [Google Scholar] [CrossRef]
- Rafi, M.M.; Vastano, B.C.; Zhu, N.; Ho, C.T.; Ghai, G.; Rosen, R.T.; Gallo, M.A.; DiPaola, R.S. Novel polyphenol molecule isolated from licorice root (Glycrrhiza glabra) induces apoptosis, G2/M cell cycle arrest, and Bcl-2 phosphorylation in tumor cell lines. J. Agric. Food Chem. 2002, 50, 677–684. [Google Scholar] [CrossRef]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef]
- van den Heuvel, S. Cell-cycle regulation. WormBook 2005, 1–16. [Google Scholar] [CrossRef]
- Morgan, D.O. Principles of CDK regulation. Nature 1995, 374, 131–134. [Google Scholar] [CrossRef]
- Arata, Y.; Takagi, H. Quantitative Studies for Cell-Division Cycle Control. Front. Physiol. 2019, 10, 1022. [Google Scholar] [CrossRef] [Green Version]
- Sperka, T.; Wang, J.; Rudolph, K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.B.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, Y.; Ziv, Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.L.; Southgate, H.; Tweddle, D.A.; Curtin, N.J. DNA damage checkpoint kinases in cancer. Expert Rev. Mol. Med. 2020, 22, e2. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Yang, J.; Li, X. The Effects of the Honey-Roasting Process on the Pharmacokinetics of the Six Active Compounds of Licorice. Evid. -Based Complement. Altern. Med. 2018, 2018, 5731276. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-Y.; Wu, T.-H.; Tzou, R.-D.; Hsu, Y.-C.; Lee, K.-T.; Tsai, T.-H. Radix Glycyrrhizae Preparata Induces Cell Cycle Arrest and Induced Caspase-Dependent Apoptosis in Glioblastoma Multiforme. Neurol. Int. 2022, 14, 804-823. https://0-doi-org.brum.beds.ac.uk/10.3390/neurolint14040066
Lin T-Y, Wu T-H, Tzou R-D, Hsu Y-C, Lee K-T, Tsai T-H. Radix Glycyrrhizae Preparata Induces Cell Cycle Arrest and Induced Caspase-Dependent Apoptosis in Glioblastoma Multiforme. Neurology International. 2022; 14(4):804-823. https://0-doi-org.brum.beds.ac.uk/10.3390/neurolint14040066
Chicago/Turabian StyleLin, Tsung-Ying, Tung-Hsuan Wu, Rong-Dar Tzou, Yi-Chiang Hsu, Kuan-Ting Lee, and Tai-Hsin Tsai. 2022. "Radix Glycyrrhizae Preparata Induces Cell Cycle Arrest and Induced Caspase-Dependent Apoptosis in Glioblastoma Multiforme" Neurology International 14, no. 4: 804-823. https://0-doi-org.brum.beds.ac.uk/10.3390/neurolint14040066