What Is the Correct Way to Manage Children Requiring Gastrostomy? Single Center Experience
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McSweeney, M.E.; Jiang, H.; Deutsch, A.J.; Atmadja, M.; Lightdale, J.R. Long-term outcomes of infants and children undergoing percutaneous endoscopy gastrostomy tube placement. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 663–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogh, B.; Kovács, T.; Saxena, A.K. Complications in children with percutaneous endoscopic gastrostomy (PEG) placement. World J. Pediatr. 2019, 15, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Gauderer, M.W.; Ponsky, J.L.; Izant, R.J., Jr. Gastrostomy without laparoscopy: A percutaneous endoscopic technique. J. Pediatr. Surg. 1980, 15, 872–875. [Google Scholar] [CrossRef]
- Sandberg, F.; Viktorsdóttir, M.B.; Salö, M.; Stenström, P.; Arnbjörnsson, E. Comparison of major complications in children after laparoscopy-assisted gastrostomy and percutaneous endoscopic gastrostomy placement: A meta-analysis. Pediatr. Surg. Int. 2018, 34, 1321–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noviello, C.; Romano, M.; Mariscoli, F.; De Benedictis, F.M.; Martino, A.; Cobellis, G. Esophageal multichannel intraluminal impedance and pH monitoring in children: Indications and limits. Minerva Pediatr. 2014, 66, 287–291. [Google Scholar] [PubMed]
- Haider, F.; Isa, H.M.A.; Al Awadhi, M.A.; Ayoub, B.; Bakhsh, E.; Al Aradi, H.; Juma, S.A. Button Gastrostomy Tubes for Pediatric Patients: A Tertiary Care Center Experience. Int. J. Pediatr. 2020, 2020, 5286283. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.R.; Kim, W.; Eun, B.L.; Shim, J.O. Percutaneous Endoscopic Gastrostomy and Nutritional Interventions by the Pediatric Nutritional Support Team Improve the Nutritional Status of Neurologically Impaired Children. J. Clin. Med. 2020, 9, 3295. [Google Scholar] [CrossRef] [PubMed]
- Nobile, S.; Noviello, C.; Cobellis, G.; Carnielli, V.P. Are Infants with Bronchopulmonary Dysplasia Prone to Gastroesophageal Reflux? A Prospective Observational Study with Esophageal pH-Impedance Monitoring. J. Pediatr. 2015, 167, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Nobile, S.; Meneghin, F.; Marchionni, P.; Noviello, C.; Salvatore, S.; Lista, G.; Carnielli, V.P.; Vento, G. Response to therapy among neonates with gastro-esophageal reflux is associated with esophageal clearance. Early. Hum. Dev. 2020, 152, 105248. [Google Scholar] [CrossRef] [PubMed]
- Yap, B.K.; Nah, S.A.; Chen, Y.; Low, Y. Fundoplication with gastrostomy vs gastrostomy alone: A systematic review and meta-analysis of outcomes and complications. Pediatr. Surg. Int. 2017, 33, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.R.; Harig, J.M.; Kozarek, R.A.; Kelsey, P.B.; Picha, G.J. Placement of a feeding button (“one-step button”) as the initial procedure. Am. J. Gastroenterol. 1993, 88, 501–504. [Google Scholar] [PubMed]
- Stone, B.; Hester, G.; Jackson, D.; Richardson, T.; Hall, M.; Gouripeddi, R.; Butcher, R.; Keren, R.; Srivastava, R. Effectiveness of Fundoplication or Gastrojejunal Feeding in Children with Neurologic Impairment. Hosp. Pediatr. 2017, 7, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noviello, C.; Romano, M.; Rossi, L.; Cobellis, G.; Martino, A. How replace the gastrostomy tube in children with extremely compromised general conditions. Afr. J. Paediatr. Surg. 2019, 16, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, L.I.; McGuire, A.; Calderon, T.; Wolcott, K.; Levatino, E.; Martin, H.; Foito, T.; Pulhamus, M.; Wakeman, D.S. Emergency department utilization following pediatric gastrostomy tube placement is driven by a small cohort of patients. J. Pediatr. Surg. 2021, 56, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Backman, E.; Sjögreen, L. Gastrostomy tube insertion in children with developmental or acquired disorders: A register-based study. Dev. Med. Child. Neurol. 2020, 62, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, G.; Torino, G.; Noviello, C.; Cruccetti, A.; Mastroianni, L.; Amici, G.; Martino, A. Versatility of one-trocar surgery in children. J. Laparoendosc. Adv. Surg. Tech. A 2011, 21, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Akay, B.; Capizzani, T.R.; Lee, A.M.; Drongowski, R.A.; Geiger, J.D.; Hirschl, R.B.; Mychaliska, G.B. Gastrostomy tube placement in infants and children: Is there a preferred technique? J. Pediatr. Surg. 2010, 45, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.R.; Lee, D.R.; Suresh, D.; Wright, H.; Upadhyaya, M.; Yardley, I.E. Open Primary Button vs. Laparoscopic Percutaneous Endoscopic Gastrostomy: Results from a Case-Control Study. J. Pediatr. Gastroenterol. Nutr. 2020, 72, e4–e9. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.T.; Balduyck, B.; Nour, S. Gastrostomy complications in infants and children: A comparative study. Pediatr. Surg. Int. 2010, 26, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Dekonenko, C.; Svetanoff, W.J.; Osuchukwu, O.O.; Pierce, A.L.; Orrick, B.A.; Sayers, K.L.; Rentea, R.M.; Aguayo, P.; Fraser, J.D.; Juang, D.; et al. Same-day discharge for pediatric laparoscopic gastrostomy. J. Pediatr. Surg. 2020, 56, 26–29. [Google Scholar] [CrossRef] [PubMed]
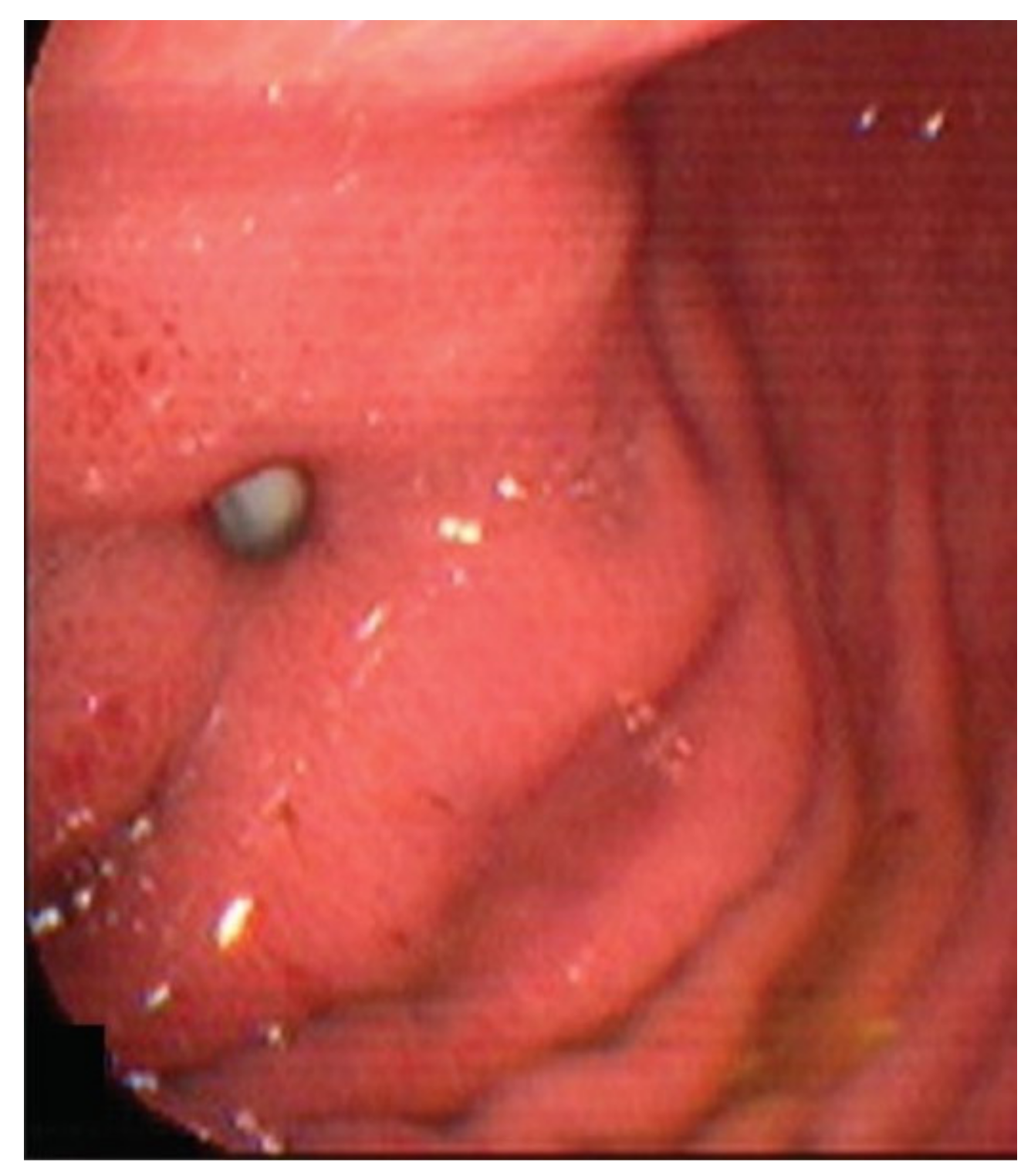
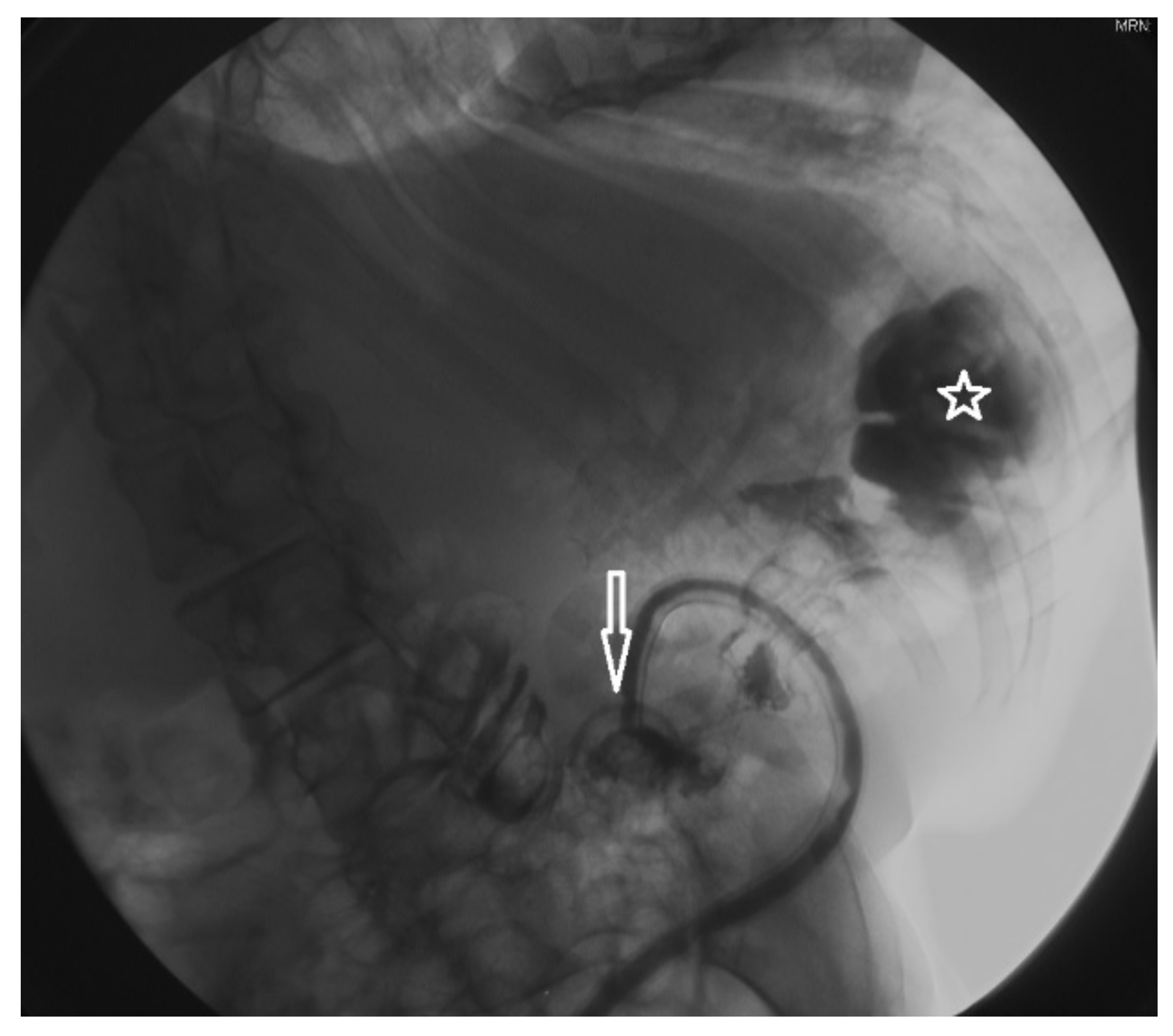
| Symptoms | Case | (%) | |
|---|---|---|---|
| Chronic inadequate oral intake | 144 | (90.6) | |
| • Inability to swallow | 30 | ||
| • Dysphagia | 34 | ||
| • Recurrent respiratory tract infections | 25 | ||
| • Vomit | 55 | ||
| Ketogenic diet support | 4 | (2.5) | |
| Impossible oral intake | 11 | (6.9) | |
| • Esophageal atresia | |||
| Pathology | Case | (%) |
|---|---|---|
| Neurologically impaired children | 111 | (75) |
| Complex heart disease | 18 | (12) |
| Metabolic disease | 10 | (6) |
| • Mucopolysaccharidosis | 4 | |
| • Niemann-pick disease | 2 | |
| • Nonketotic hyperglycinemia | 2 | |
| • Unspecified metabolic disease | 2 | |
| Cystic fibrosis | 4 | (3) |
| Muscle disease | 4 | (3) |
| • Congenital muscular dystrophy | ||
| Chromosomopathy | 1 | |
| • Trisomy 18 syndrome |
| Complication | Peg | Laparo-assisted | Open | Case (%) |
| N° of cases operated | 101 | 44 | 3 | |
| Major | 10 | 3 | 0 | 13 (8.7) |
| • Infections with gastrostomy dislocation | 4 | 3 | 0 | 1 |
| • Bumper retractions | 5 | 0 | 0 | 5 |
| • Gastrocolic fistula | 1 | 0 | 0 | 7 |
| Minor | 42 | 19 | 1 | 62 (42.9) |
| • Peristomal granuloma | 31 | 10 | 0 | 41 |
| • Wound infections | 5 | 4 | 1 | 10 |
| • Peristomal wound leakage | 6 | 5 | 0 | 11 |
| Complication | |||
| Age < 24 months | Age> 24 months | Case (%) | |
| N° of cases operated | 63 | 85 | |
| Major | 4 | 9 | 13 (8.7) |
| • Infections with gastrostomy dislocation | 3 | 4 | |
| • Bumper retractions | 1 | 4 | |
| • Gastrocolic fistula | 0 | 1 | |
| Minor | 21 | 41 | 62 (42.9) |
| • Peristomal granuloma | 10 | 25 | 41 |
| • Wound infections | 5 | 11 | 10 |
| • Peristomal wound leakage | 6 | 5 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noviello, C.; Romano, M.; Bindi, E.; Cobellis, G.; Nobile, S.; Papparella, A. What Is the Correct Way to Manage Children Requiring Gastrostomy? Single Center Experience. Gastroenterol. Insights 2021, 12, 329-335. https://0-doi-org.brum.beds.ac.uk/10.3390/gastroent12030030
Noviello C, Romano M, Bindi E, Cobellis G, Nobile S, Papparella A. What Is the Correct Way to Manage Children Requiring Gastrostomy? Single Center Experience. Gastroenterology Insights. 2021; 12(3):329-335. https://0-doi-org.brum.beds.ac.uk/10.3390/gastroent12030030
Chicago/Turabian StyleNoviello, Carmine, Mercedes Romano, Edoardo Bindi, Giovanni Cobellis, Stefano Nobile, and Alfonso Papparella. 2021. "What Is the Correct Way to Manage Children Requiring Gastrostomy? Single Center Experience" Gastroenterology Insights 12, no. 3: 329-335. https://0-doi-org.brum.beds.ac.uk/10.3390/gastroent12030030







