Novel Low-Alcohol Sangria-Type Wine Products with Immobilized Kefir Cultures and Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
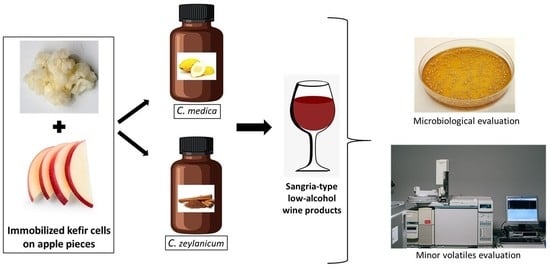
2.1. Kefir Culture Immobilization and Production of Dried Cultures
2.2. Novel Low-Alcohol Sangria-Type Wines Preparation
2.3. Microbial Enumeration
2.4. Chemical Analyses
2.5. Preliminary Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Viability of Immobilized Kefir Cultures in the Novel Sangria-Type Low-Alcohol Wines
3.2. Chemical Analyses
3.2.1. pH and Acidity Values
3.2.2. HS-SPME GC/MS Analyses
3.2.3. Principal Component Analysis
3.3. Preliminary Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast’s balancing act between ethanol and glycerol production in low-alcohol wines. Microb. Biotechnol. 2017, 10, 264–278. [Google Scholar] [CrossRef]
- Nikolaou, A.; Sgouros, G.; Mitropoulou, G.; Santarmaki, V.; Kourkoutas, Y. Freeze-dried Immobilized Kefir Culture in Low-Alcohol Wine-Making. Foods 2020, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Nikolaou, A.; Sgouros, G.; Santarmaki, V.; Mitropoulou, G.; Kourkoutas, Y. Preliminary Evaluation of the Use of Thermally-Dried Immobilized Kefir Cells in Low Alcohol Winemaking. Appl. Sci. 2022, 12, 6176. [Google Scholar] [CrossRef]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological Exploration of Different Types of Kefir Grains. Fermentation 2017, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Kumura, H.; Tanoue, Y.; Tsukahara, M.; Tanaka, T.; Shimazaki, K. Screening of dairy yeast strains for probiotic applications. J. Dairy Sci. 2004, 87, 4050–4056. [Google Scholar] [CrossRef] [Green Version]
- Tas, T.K.; Ekinci, F.Y.; Guzel-Seydim, Z.B. Identification of microbial flora in kefir grains produced in Turkey using PCR. Int. J. Dairy Technol. 2012, 65, 126–131. [Google Scholar] [CrossRef]
- Roberfroid, M. 1—Defining functional foods and associated claims. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Functional Foods, 2nd ed.; Saarela, M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–24. [Google Scholar] [CrossRef]
- Ribeiro, A.P.D.O.; Gomes, F.D.S.; dos Santos, K.M.O.; da Matta, V.M.; de Sá, D.D.G.C.F.; Santiago, M.C.P.D.A.; Conte, C.; Costa, S.D.D.O.; Ribero, L.D.O.; Godoy, R.L.D.O.; et al. Development of a probiotic non-fermented blend beverage with juçara fruit: Effect of the matrix on probiotic viability and survival to the gastrointestinal tract. LWT 2020, 118, 108756. [Google Scholar] [CrossRef]
- Bekatorou, A.; Plessas, S.; Mallouchos, A. Cell Immobilization Technologies for Applications in Alcoholic Beverages. In Applications of Encapsulation and Controlled Release, 1st ed.; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 933–955. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Santarmaki, V.; Nikolaou, A.; Mitropoulou, G.; Kandylis, P.; Kourkoutas, Y. Encapsulated/Immobilized Biocatalysts for Production of Dairy Products. In Encapsulation in Food Processing and Fermentation, 1st ed.; Lević, S., Nedović, V., Bugarski, B., Eds.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Tristezza, M.; Lourenço, A.; Barata, A.; Brito, L.; Malfeito-Ferreira, M.; Loureiro, V. Susceptibility of wine spoilage yeasts and bacteria in the planktonic state and in biofilms to disinfectants. Ann. Microbiol. 2010, 60, 549–556. [Google Scholar] [CrossRef]
- Enrique, M.; Marcos, J.F.; Yuste, M.; Martínez, M.; Vallés, S.; Manzanares, P. Antimicrobial action of synthetic peptides towards wine spoilage yeasts. Int. J. Food Microbiol. 2007, 118, 318–325. [Google Scholar] [CrossRef]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef]
- Cavaglia, J.; Mas Garcia, S.; Roger, J.-M.; Mestres, M.; Boqué, R. Detection of bacterial spoilage during wine alcoholic fermentation using ATR-MIR and MCR-ALS. Food Control 2022, 142, 109269. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Abramovič, H.; Košmerl, T.; Poklar Ulrih, N.; Cigić, B. Contribution of SO2 to antioxidant potential of white wine. Food Chem. 2015, 174, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Caldeira, J.; Botelheiro, R.; Pagliara, D.; Malfeito-Ferreira, M.; Loureiro, V. Survival patterns of Dekkera bruxellensis in wines and inhibitory effect of sulphur dioxide. Int. J. Food Microbiol. 2008, 121, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Siebert, T.E.; Solomon, M.R.; Pollnitz, A.P.; Jeffery, D.W. Selective Determination of Volatile Sulfur Compounds in Wine by Gas Chromatography with Sulfur Chemiluminescence Detection. Agric. Food Chem. 2010, 58, 9454–9462. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L. Adverse reactions to the sulphite additives. Gastroenterol. Hepatol. Bed Bench. 2012, 5, 16–23. [Google Scholar] [PubMed]
- Gupta, M.K.; Basavaraj, G.V. Sulphites in food & drinks in asthmatic adults & children: What we need to know. Indian J. Allergy Asthma Immunol. 2021, 35, 43–47. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Garcia-Sotelo, D.; Silva-Espinoza, B.; Perez-Tello, M.; Olivas, I.; Alvarez-Parrilla, E.; González-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial activity and thermal stability of rosemary essential oil:β−cyclodextrin capsules applied in tomato juice. LWT Food Sci. Technol. 2019, 111, 837–845. [Google Scholar] [CrossRef]
- Arasu, M.V.; Viayaraghavan, P.; Ilavenil, S.; Al-Dhabi, N.A.; Choi, K.C. Essential oil of four medicinal plants and protective properties in plum fruits against the spoilage bacteria and fungi. Ind. Crops Prod. 2019, 133, 54–62. [Google Scholar] [CrossRef]
- Demirok Soncu, E.; Özdemir, N.; Arslan, B.; Küçükkaya, S.; Soyer, A. Contribution of surface application of chitosan-thyme and chitosan-rosemary essential oils to the volatile composition, microbial profile, and physicochemical and sensory quality of dry-fermented sausages during storage. Meat Sci. 2020, 166, 108127. [Google Scholar] [CrossRef]
- Milanović, V.; Sabbatini, R.; Garofalo, C.; Cardinali, F.; Pasquini, M.; Aquilanti, L.; Osimani, A. Evaluation of the inhibitory activity of essential oils against spoilage yeasts and their potential application in yogurt. Int. J. Food Microbiol. 2021, 341, 109048. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Mitropoulou, G.; Nikolaou, A.; Santarmaki, V.; Sgouros, G.; Kourkoutas, Y. Citrus medica and Cinnamomum zeylanicum Essential Oils as Potential Biopreservatives against Spoilage in Low Alcohol Wine Products. Foods 2020, 9, 577. [Google Scholar] [CrossRef]
- Ganić, T.; Vuletić, S.; Nikolić, B.; Stevanović, M.; Kuzmanović, M.; Kekić, D.; Đurović, S.; Cvetković, S.; Mitić-Ćulafić, D. Cinnamon essential oil and its emulsion as efficient antibiofilm agents to combat Acinetobacter baumannii. Front. Microbiol. 2022, 13, 989667. [Google Scholar] [CrossRef]
- Arachchige, S.P.G.; Abeysekera, W.P.K.M.; Ratnasooriya, W.D. Antiamylase, Anticholinesterases, Antiglycation, and Glycation Reversing Potential of Bark and Leaf of Ceylon Cinnamon (Cinnamomum zeylanicum Blume) In Vitro. Evid. Based Complement. Alternat. Med. 2017, 2017, 5076029. [Google Scholar] [CrossRef] [Green Version]
- Abeysekera, W.P.K.M.; Premakumara, G.A.S.; Ratnasooriya, W.D.; Abeysekera, W.K.S.M. Anti-inflammatory, cytotoxicity and antilipidemic properties: Novel bioactivities of true cinnamon (Cinnamomum zeylanicum Blume) leaf. BMC Complement. Med. Ther. 2022, 22, 259. [Google Scholar] [CrossRef]
- Singh, R.; Parasuraman, S.; Kathiresan, S. Antioxidant and Antidiabetic Activity of Methanolic extract of Bark of Cinnamomum zeylanicum in Diabetic Rats. Free Radic. Antioxid. 2020, 10, 16–23. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Fitsiou, E.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Bardouki, H.; Vamvakias, M.; Panas, P.; Chlichlia, K.; Pappa, A.; Kourkoutas, Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT-Food Sci. Technol. 2017, 84, 344–352. [Google Scholar] [CrossRef]
- Ghani, A.; Taghvaeefard, N.; Hosseinifarahi, M.; Dakhlaoui, S.; Msaada, K. Essential oil composition and antioxidant activity of citron fruit (Citrus medica var. macrocarpa Risso.) peel as relation to ripening stages. Int. J. Environ. Health Res. 2022, 18, 1–11. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; de Cindio, B.; Loizzo, M.R.; Conforti, F.; Statti, G.A.; Menabeni, R.; Bettini, R.; Menichini, F. Chemical composition and bioactivity of Citrus medica L. cv. Diamante essential oil obtained by hydrodistillation, cold-pressing and supercritical carbon dioxide extraction. Nat. Prod. Res. 2011, 25, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Panara, K.; Joshi, K.; Nishteswar, K. A Review on Phytochemical and Pharmacological Properties of Citrus medica Linn. Int. J. Pharm. Biol. 2012, 3, 1292–1297. [Google Scholar]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef] [Green Version]
- Aminzare, M.; Aliakbarlu, J.; Tajik, H. The effect of Cinnamomum zeylanicum essential oil on chemical characteristics of Lyoner- type sausage during refrigerated storage. Vet. Res. Forum 2015, 6, 31–39, PMCID:4405683. [Google Scholar] [PubMed]
- Salama, H.H.; El-Sayed, H.S.; Kholif, A.M.M.; Edris, A.E. Essential oils nanoemulsion for the flavoring of functional stirred yogurt: Manufacturing, physicochemical, microbiological, and sensorial investigation. J. Saudi Soc. Agric. Sci. 2022, 21, 372–382. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of Some Citrus Essential Oils on Post-Harvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Brnawi, W.I.; Hettiarachchy, N.S.; Horax, R.; Kumar-Phillips, G.; Seo, H.S.; Marcy, J. Comparison of Cinnamon Essential Oils from Leaf and Bark with Respect to Antimicrobial Activity and Sensory Acceptability in Strawberry Shake. J. Food Sci. 2018, 83, 475–480. [Google Scholar] [CrossRef]
- Rashid, Z.; Khan, M.R.; Mubeen, R.; Hassan, A.; Saeed, F.; Afzaal, M. Exploring the effect of cinnamon essential oil to enhance the stability and safety of fresh apples. J. Food Process. Preserv. 2020, 44, e14926. [Google Scholar] [CrossRef]
- Nelios, G.; Santarmaki, V.; Pavlatou, C.; Dimitrellou, D.; Kourkoutas, Y. New Wild-Type Lacticaseibacillus rhamnosus Strains as Candidates to Manage Type 1 Diabetes. Microorganisms 2022, 10, 272. [Google Scholar] [CrossRef]
- Nikolaou, A.; Tsakiris, A.; Kanellaki, M.; Bezirtzoglou, E.; Akrida-Demertzi, K.; Kourkoutas, Y. Wine production using free and immobilized kefir culture on natural supports. Food Chem. 2019, 272, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, A.; Kourkoutas, Y. High-Temperature Semi-Dry and Sweet Low Alcohol Wine-Making Using Immobilized Kefir Culture. Fermentation 2021, 7, 45. [Google Scholar] [CrossRef]
- Nikolaou, A.; Galanis, A.; Kanellaki, M.; Tassou, C.; Akrida-Demertzi, K.; Kourkoutas, Y. Assessment of free and immobilized kefir culture in simultaneous alcoholic and malolactic cider fermentations. LWT Food Sci. Technol. 2017, 76, 67–78. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Calabuig-Jiménez, L.; Barrera, C.; Dalla Rosa, M. Effect of Drying Process, Encapsulation, and Storage on the Survival Rates and Gastrointestinal Resistance of L. salivarius spp. salivarius Included into a Fruit Matrix. Microorganisms 2020, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Haindl, R.; Neumayr, A.; Frey, A.; Kulozik, U. Impact of cultivation strategy, freeze-drying process, and storage conditions on survival, membrane integrity, and inactivation kinetics of Bifidobacterium longum. Folia Microbiol. 2020, 65, 1039–1050. [Google Scholar] [CrossRef]
- Mileriene, J.; Serniene, L.; Kondrotiene, K.; Santarmaki, V.; Kourkoutas, Y.; Vasiliauskaite, A.; Lauciene, L.; Malakauskas, M. Indigenous Lactococcus lactis with Probiotic Properties: Evaluation of Wet, Thermally- and Freeze-Dried Raisins as Supports for Cell Immobilization, Viability and Aromatic Profile in Fresh Curd Cheese. Foods 2022, 11, 1311. [Google Scholar] [CrossRef]
- Tomova, T.; Petelkov, I.; Shopska, V.; Denkova-Kostova, R.; Kostov, G.; Denkova, Z. Production of probiotic wort-based beverages with grapefruit (Citrus paradisi L.) or tangerine (Citrus reticulata L.) zest essential oil addition. Acta Sci. Pol. Technol. Aliment. 2021, 20, 237–245. [Google Scholar] [CrossRef]
- Teneva, D.; Denkova, Z.; Denkova-Kostova, R.; Goranov, B.; Kostov, G.; Slavchev, A.; Hristova-Ivanova, Y.; Uzunova, G.; Degraeve, P. Biological preservation of mayonnaise with Lactobacillus plantarum LBRZ12, dill, and basil essential oils. Food Chem. 2021, 344, 128707. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [Green Version]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.S.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbialcultures, biologicalactivities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef] [Green Version]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of Health Benefits Conferred by Lactobacillus Species from Kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carasi, P.; Ambrosis, N.M.; De Antoni, G.L.; Bressollier, P.; Urdaci, M.C.; Serradell, M.L. Adhesion properties of potentially probiotic Lactobacillus kefiri to gastrointestinal mucus. J. Dairy Res. 2014, 81, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Zavala, L.; Golowczyc, M.A.; van Hoorde, K.; Medrano, M.; Huys, G.; Vandamme, P.; Abraham, A.G. Selected Lactobacillus strains isolated from sugary and milk kefir reduce Salmonella infection of epithelial cells in vitro. Benef. Microbes 2016, 7, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jeong, D.; Kang, I.B.; Kim, H.; Song, K.Y.; Seo, K.H. Dual function of Lactobacillus kefiri DH5 in preventing high-fat-diet-induced obesity: Direct reduction of cholesterol and upregulation of PPAR-α in adipose tissue. Mol. Nutr. Food Res. 2017, 61, 1700252. [Google Scholar] [CrossRef] [PubMed]
- Zubiría, M.G.; Gambaro, S.E.; Rey, M.A.; Carasi, P.; Serradell, M.L.A.; Giovambattista, A. Deleterious Metabolic Effects of High Fructose Intake: The Preventive Effect of Lactobacillus kefiri Administration. Nutrients 2017, 9, 470. [Google Scholar] [CrossRef]
- Carasi, P.; Racedo, S.M.; Jacquot, C.; Romanin, D.E.; Serradell, M.A.; Urdaci, M.C. Impact of kefir derived Lactobacillus kefiri on the mucosal immune response and gut microbiota. J. Immuno. Res. 2015, 2015, 361604. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xie, Y.; Xiong, L.; Dong, R.; Pan, C.; Teng, G.; Zhang, H. Effect and Mechanism of Cholesterol-Lowering by Kluyveromyces from Tibetan Kefir. Adv. Mat. Res. 2012, 343, 1290–1298. [Google Scholar] [CrossRef]
- Maccaferri, S.; Klinder, A.; Brigidi, P.; Cavina, P.; Costabile, A. Potential probiotic Kluyveromyces marxianus B0399 modulates the immune response in Caco-2 cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system. Appl. Environ. Microbiol. 2012, 78, 956–964. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.D.S.F.; Souza, K.M.S.; Albuquerque, W.W.C.; Teixeira, J.A.C.; Cavalcanti, M.T.H.; Porto, A.L.F. Saccharomyces cerevisiae from Brazilian kefir-fermented milk: An in vitro evaluation of probiotic properties. Microb. Pathog. 2017, 110, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.R.; Lopes, A.C.A.; Pereira, R.A.; Cardoso, P.G.; Duarte, W.F. Selection of potentially probiotic Kluyveromyces lactis for the fermentation of cheese whey–based beverage. Ann. Microbiol. 2019, 69, 1361–1372. [Google Scholar] [CrossRef]
- Mantzari, E.; Marteau, T.M. Impact of Sizes of Servings, Glasses and Bottles on Alcohol Consumption: A Narrative Review. Nutrients 2022, 14, 4244. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.-J.; Kim, D.-H.; Jeong, D.; Seo, K.-H.; Jeong, H.S.; Lee, H.G.; Kim, H. Characterization of yeasts isolated from kefir as a probiotic and its synergic interaction with the wine byproduct grape seed flour/extract. LWT Food Sci. Technol. 2018, 9, 535–539. [Google Scholar] [CrossRef]
- Coimbra, A.; Ferreira, S.; Duarte, A.P. Biological properties of Thymus zygis essential oil with emphasis on antimicrobial activity and food application. Food Chem. 2022, 393, 133370. [Google Scholar] [CrossRef]
- Mith, H.; Duré, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, K.-P.; Tu, S.-H.; Su, Y.-C.; Ho, C.-L. Chemical Composition and Antimicrobial Activity Against Food-Borne Pathogens of Calocedrus formosana Heartwood Essential Oil. Natl. Prod. Commun. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Park, J.-B.; Kang, J.-H.; Song, K.B. Antibacterial activities of a cinnamon essential oil with cetylpyridinium chloride emulsion against Escherichia coli O157:H7 and Salmonella Typhimurium in basil leaves. Food Sci. Biotechnol. 2018, 27, 47–55. [Google Scholar] [CrossRef]
- Ahmed, Z.; Wang, Y.; Ahmad, A.; Khan, S.T.; Nisa, M.; Ahmad, H.; Afreen, A. Kefir and health: A contemporary perspective. Crit. Rev. Food Sci. Nutr. 2013, 53, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science: Principles and Applications, 4th ed.; Academic Press Inc.: San Diego, CA, USA, 2014. [Google Scholar]
- Boss, P.K.; Pearce, A.D.; Zhao, Y.; Nicholson, E.L.; Dennis, E.G.; Jeffery, D.W. Potential grape-derived contributions to volatile ester concentrations in wine. Molecules 2015, 20, 7845–7873. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zhu, B.-Q.; Wang, Y.-H.; Lu, L.; Lan, Y.-B.; Reeves, M.J.; Duan, C.-Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Du, Q.; Qu, R.; Ye, D.; Lu, Y.; Liu, Y. Analysis of Volatile Aroma Compounds and Sensory Characteristics Contributing to Regional Style of Red Wines from Hexi Corridor Based on Sixteen Grape Varieties/Clones. Fermentation 2022, 8, 501. [Google Scholar] [CrossRef]
- Gotmare, S.; Tambe, E. Identification of Chemical Constituents of Cinnamon Bark Oil by GCMS and Comparative Study Garnered from Five Different Countries. Glob. J. Sci. Front. Res. Biol. Sci. 2019, 19, 35–42. [Google Scholar]
- Pino, J.A.; Queris, O. Characterization of odor-active compounds in guava wine. J. Agric. Food Chem. 2011, 59, 4885–4890. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ho, C.-T.; Wan, X.; Zhu, H.; Liu, Q.; Wen, Z. Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chem. 2021, 341, 128230. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Mo, Y.; Chen, D.; Feng, T.; Song, S.; Wang, H.; Sun, M. Characterization of key aroma compounds in Xinjiang dried figs (Ficus carica L.) by GC–MS, GC–olfactometry, odor activity values, and sensory analyses. LWT Food Sci. Technol. 2021, 150, 111982. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, K.; Xu, Y.; Thomas-Danguin, T. A dataset on odor intensity and odor pleasantness of 222 binary mixtures of 72 key food odorants rated by a sensory panel of 30 trained assessors. Data Brief. 2021, 36, 107143. [Google Scholar] [CrossRef]
- Schmidt, E.; Bail, S.; Buchbauer, G.; Stoilova, I.; Krastanov, A.; Stoyanova, A.; Jirovetz, L. Chemical Composition, Olfactory Evaluation and Antioxidant Effects of the Essential oil of Origanum Majorana L. from Albania. Nat. Prod. Commun. 2008, 3, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Fan, B.; Niu, Y.; Wu, M.; Liu, J.; Ma, S. Characterization of odor-active compounds of various Chrysanthemum essential oils by gas chromatography-olfactometry, gas chromatography-mass spectrometry and their correlation with sensory attributes. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1009–1010, 152–162. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.B.; Romani, A.; Lampe, A.; Ferrari Nicoli, S.; Gabrielli, P.; et al. Grape Seeds: Chromatographic Profile of Fatty Acids and Phenolic Compounds and Qualitative Analysis by FTIR-ATR Spectroscopy. Foods 2020, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Šikuten, I.; Štambuk, P.; Tomaz, I.; Marchal, C.; Kontić, J.K.; Lacombe, T.; Maletić, E.; Preiner, D. Discrimination of genetic and geographical groups of grape varieties (Vitis vinifera L.) based on their volatile organic compounds. Front. Plant. Sci. 2022, 13, 942148. [Google Scholar] [CrossRef]
- Dunlevy, J.; Kalua, C.; Keyzers, R.; Boss, P. The Production of Flavour & Aroma Compounds in Grape Berries. In Grapevine Molecular Physiology & Biotechnology, 2nd ed.; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 293–340. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhang, Y.; Qiu, J.; Cao, J.; Sun, Y.; Li, H.; Kong, F. Coupled multidimensional GC and odor activity value calculation to identify off-odors in thermally processed muskmelon juice. Food Chem. 2019, 301, 125307. [Google Scholar] [CrossRef]
- Bartsch, J.; Uhde, E.; Salthammer, T. Analysis of odour compounds from scented consumer products using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Anal. Chim. Acta 2016, 904, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. The microbiology of wine and vinifications. In Handbook of Enology; Wiley: Chichester, UK, 2006; Volume 1. [Google Scholar]
- Niu, Y.; Yao, Z.; Xiao, Z.; Zhu, G.; Zhu, J.; Chen, J. Sensory evaluation of the synergism among ester odorants in light aroma-type liquor by odor threshold, aroma intensity and flash GC electronic nose. Food Res. Int. 2018, 113, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 251/2014 of the European Parliament and of the Council of 26 February 2014 on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Aromatised Wine Products and Repealing Council Regulation (EEC) No 1601/91. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:32014R0251 (accessed on 23 February 2023).
- Sharif, M.; Butt, M.; Sharif, H.; Nasir, M. Sensory Evaluation and Consumer Acceptability. In Handbook of Food Science and Technology; Wiley: Hoboken, NJ, USA, 2017; pp. 362–386. [Google Scholar]
- Krishna, A. An integrative review of sensory marketing: Engaging the senses to affect perception, judgment and behavior. J. Consum. Psychol. 2012, 22, 332–351. [Google Scholar] [CrossRef] [Green Version]



| Wine Sample | Total Acidity (g Tartaric/L) | Volatile Acidity (g Acetic/L) | pH | Alcohol (% vol) | |
|---|---|---|---|---|---|
| Wet kefir cultures | Co_Wt_Ap | 2.6 ± 0.1 | 0.54 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 |
| Co_Wt_Pe | 2.1 ± 0.1 | 0.51 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Cm_Wt_Ap | 2.0 ± 0.1 | 0.57 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Cm_Wt_Pe | 1.8 ± 0.1 | 0.51 ± 0.02 | 4.0 ± 0.1 | 6.0 ± 0.1 | |
| Cz_Wt_Ap | 2.1 ± 0.1 | 0.54 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Cz_Wt_Pe | 2.3 ± 0.1 | 0.54 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Mix_Wt_Ap | 2.6 ± 0.1 | 0.57 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Mix_Wt_Pe | 2.0 ± 0.1 | 0.54 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Thermally dried kefir cultures | Co_ThDr_Ap | 2.3 ± 0.1 | 0.48 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 |
| Co_ThDr_Pe | 2.6 ± 0.1 | 0.51 ± 0.02 | 4.0 ± 0.1 | 6.0 ± 0.1 | |
| Cm_ThDr_Ap | 3.0 ± 0.1 | 0.63 ± 0.03 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Cm_ThDr_Pe | 2.0 ± 0.1 | 0.51 ± 0.02 | 4.0 ± 0.1 | 6.0 ± 0.1 | |
| Cz_ThDr_Ap | 2.9 ± 0.1 | 0.54 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Cz_ThDr_Pe | 2.7 ± 0.1 | 0.57 ± 0.03 | 4.0 ± 0.1 | 6.0 ± 0.1 | |
| Mix_ThDr_Ap | 3.0 ± 0.1 | 0.57 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Mix_ThDr_Pe | 2.3 ± 0.1 | 0.57 ± 0.03 | 4.0 ± 0.1 | 6.0 ± 0.1 | |
| Freeze-dried kefir cultures | Co_FrDr_Ap | 2.7 ± 0.1 | 0.45 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 |
| Co_FrDr_Pe | 2.4 ± 0.1 | 0.42 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Cm_FrDr_Ap | 2.7 ± 0.1 | 0.39 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Cm_FrDr_Pe | 2.1 ± 0.1 | 0.45 ± 0.03 | 4.0 ± 0.1 | 6.0 ± 0.1 | |
| Cz_FrDr_Ap | 2.7 ± 0.1 | 0.39 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Cz_FrDr_Pe | 2.1 ± 0.1 | 0.45 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Mix_FrDr_Ap | 2.6 ± 0.1 | 0.42 ± 0.02 | 3.9 ± 0.1 | 6.0 ± 0.1 | |
| Mix_FrDr_Pe | 2.1 ± 0.1 | 0.42 ± 0.02 | 4.0 ± 0.1 | 6.0 ± 0.1 |
| Essential Oil Addition | Samples | Compounds Detected | |||||
|---|---|---|---|---|---|---|---|
| Esters | Acids | Terpenes | Alcohols | Carbonyls | Miscellaneous | ||
| Citrus medica (0.01% v/v) | Cm_Wt_Ap | 8.4 a | 0.3 a | 89.1 f | 2.1 a | <0.1 a | 0.1 a |
| Cm_ThDr_Ap | 8.5 a | 0.3 a | 86.9 f | 3.8 a | <0.1 a | 0.4 b | |
| Cm_FrDr_Ap | 8.5 a | 0.3 a | 87.7 f | 3.4 a | <0.1 a | 0.1 a | |
| Cm_Wt_Pe | 4.9 A | 0.2 A | 92.2 D | 2.5 A | <0.1 A | 0.1 A | |
| Cm_ThDr_Pe | 3.9 A | 0.2 A,B | 92.7 D | 2.9 A | <0.1 A | 0.2 A | |
| Cm_FrDr_Pe | 12.3 B | 0.3 A,B | 84.6 D | 2.5 A | <0.1 A | 0.2 A | |
| Cinnamon zeylanicum (0.01% v/v) | Cz_Wt_Ap | 37.9 c | 1.0 b | 33.5 c | 24.8 e | 2.0 b | 0.8 c |
| Cz_ThDr_Ap | 42.4 c | 1.0 b | 24.8 b | 18.9 d | 10.9 d | 1.9 f | |
| Cz_FrDr_Ap | 36.3 c | 2.3 c | 21.7 b | 17.1 d | 21.3 f | 1.2 e | |
| Cz_Wt_Pe | 30.8 D | 1.2 D | 34.1 B | 29.1 F | 3.3 E | 1.5 E | |
| Cz_ThDr_Pe | 29.8 D | 1.8 E | 34.4 B | 25.3 E | 5.9 F | 2.8 F | |
| Cz_FrDr_Pe | 37.6 E | 1.0 C,D | 33.3 B | 16.6 D | 10.3 G | 1.2 D | |
| EO Mix (0.005% v/v each) | Mix_Wt_Ap | 23.1 b | 0.4 a | 68.1 e | 7.4 b | 0.7 a | 0.4 b |
| Mix_ThDr_Ap | 19.0 b | 0.9 b | 65.8 d,e | 7.4 b | 6.0 c | 1.0 d | |
| Mix_FrDr_Ap | 19.7 b | 1.0 b | 58.8 d | 7.5 b | 12.7 e | 0.4 b | |
| Mix_Wt_Pe | 19.0 C | 0.5 A,B,C | 72.1 C | 6.8 B | 0.9 B,C | 0.7 B,C | |
| Mix_ThDr_Pe | 11.8 B | 1.0 C,D | 74.7 C | 10.1 C | 1.2 C,D | 1.2 D | |
| Mix_FrDr_Pe | 14.6 B,C | 0.8 B,C,D | 70.3 C | 7.7 B,C | 6.0 F | 0.6 B | |
| No EO addition | Co_Wt_Ap | 68.7 d | 4.3 e | 2.9 a | 23.7 e | 0.1 a | 0.4 b |
| Co_ThDr_Ap | 76.3 e | 2.2 c | 1.1 a | 18.7 d | 0.4 a | 1.3 e | |
| Co_FrDr_Ap | 82.1 e | 3.6 d | 1.8 a | 11.4 c | 0.2 a | 0.9 c | |
| Co_Wt_Pe | 72.5 H | 2.3 F | 8.2 A | 15.7 D | 0.4 A,B | 0.8 C | |
| Co_ThDr_Pe | 59.5 G | 5.0 G | 2.5 A | 29.8 F | 1.6 D | 1.5 E | |
| Co_FrDr_Pe | 45.3 F | 10.7 H | 5.6 A | 38.1 G | 0.1 A | 0.2 A | |
| Wine Products Supplemented with EOs | Aroma Density | Taste Density | Overall Evaluation | |
|---|---|---|---|---|
| Wet kefir culture | Co_Wt_Ap | 2.8 ± 0.4 | 2.8 ± 0.4 | 3.6 ± 0.7 |
| Co_Wt_Pe | 2.7 ± 0.5 | 3.1 ± 0.2 | 3.5 ± 0.6 | |
| Cm_Wt_Ap | 3.8 ± 0.8 | 3.2 ± 0.4 | 3.3 ± 0.9 | |
| Cm_Wt_Pe | 4.0 ± 0.9 | 4.3 ± 0.5 | 2.9 ± 0.9 | |
| Cz_Wt_Ap | 3.3 ± 0.4 | 3.4 ± 0.5 | 3.3 ± 0.7 | |
| Cz_Wt_Pe | 3.5 ± 0.8 | 2.9 ± 0.2 | 3.4 ± 0.5 | |
| Mix_Wt_Ap | 4.2 ± 0.4 | 3.5 ± 0.8 | 3.3 ± 0.9 | |
| Mix_Wt_Pe | 3.5 ± 0.6 | 3.3 ± 0.5 | 3.3 ± 0.7 | |
| Thermally dried kefir culture | Co_ThDr_Ap | 2.6 ± 0.7 | 2.9 ± 0.5 | 3.0 ± 0.6 |
| Co_ThDr_Pe | 2.9 ± 0.4 | 2.8 ± 0.7 | 3.7 ± 0.8 | |
| Cm_ThDr_Ap | 3.6 ± 0.6 | 3.5 ± 0.6 | 2.6 ± 0.7 | |
| Cm_ThDr_Pe | 3.6 ± 0.6 | 3.2 ± 0.6 | 2.9 ± 0.9 | |
| Cz_ThDr_Ap | 2.9 ± 0.5 | 3.1 ± 0.8 | 3.1 ± 0.7 | |
| Cz_ThDr_Pe | 3.6 ± 0.5 | 3.4 ± 0.4 | 3.4 ± 0.6 | |
| Mix_ThDr_Ap | 3.2 ± 0.6 | 3.1 ± 0.5 | 3.0 ± 0.8 | |
| Mix_ThDr_Pe | 3.5 ± 0.7 | 3.3 ± 0.4 | 3.2 ± 0.7 | |
| Freeze-dried kefir culture | Co_FrDr_Ap | 2.6 ± 0.7 | 2.9 ± 0.5 | 3.4 ± 0.9 |
| Co_FrDr_Pe | 2.4 ± 0.7 | 2.8 ± 0.5 | 3.1 ± 0.7 | |
| Cm_FrDr_Ap | 3.4 ± 0.9 | 3.3 ± 0.6 | 2.7 ± 0.6 | |
| Cm_FrDr_Pe | 3.4 ± 0.7 | 3.7 ± 0.8 | 2.5 ± 0.9 | |
| Cz_FrDr_Ap | 3.7 ± 0.7 | 3.8 ± 0.5 | 2.8 ± 0.6 | |
| Cz_FrDr_Pe | 3.6 ± 0.7 | 3.4 ± 0.5 | 2.8 ± 0.8 | |
| Mix_FrDr_Ap | 4.2 ± 0.3 | 3.6 ± 0.8 | 3.0 ± 0.8 | |
| Mix_FrDr_Pe | 3.3 ± 0.5 | 3.3 ± 0.4 | 3.0 ± 0.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaou, A.; Santarmaki, V.; Mitropoulou, G.; Sgouros, G.; Kourkoutas, Y. Novel Low-Alcohol Sangria-Type Wine Products with Immobilized Kefir Cultures and Essential Oils. Microbiol. Res. 2023, 14, 543-558. https://0-doi-org.brum.beds.ac.uk/10.3390/microbiolres14020038
Nikolaou A, Santarmaki V, Mitropoulou G, Sgouros G, Kourkoutas Y. Novel Low-Alcohol Sangria-Type Wine Products with Immobilized Kefir Cultures and Essential Oils. Microbiology Research. 2023; 14(2):543-558. https://0-doi-org.brum.beds.ac.uk/10.3390/microbiolres14020038
Chicago/Turabian StyleNikolaou, Anastasios, Valentini Santarmaki, Gregoria Mitropoulou, Georgios Sgouros, and Yiannis Kourkoutas. 2023. "Novel Low-Alcohol Sangria-Type Wine Products with Immobilized Kefir Cultures and Essential Oils" Microbiology Research 14, no. 2: 543-558. https://0-doi-org.brum.beds.ac.uk/10.3390/microbiolres14020038