“Predator-In-First”: A Preemptive Biological Control Strategy for Sustainable Management of Pepper Pests in Florida
Abstract
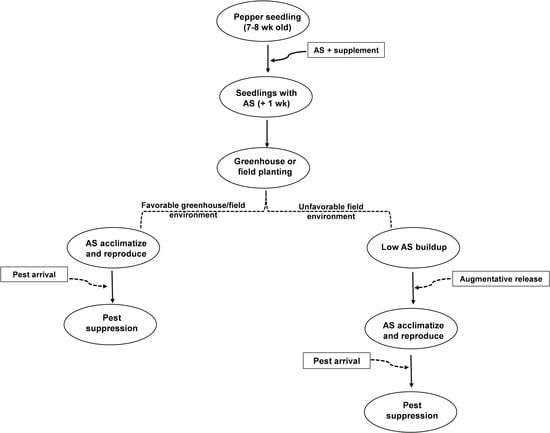
:1. Introduction
2. Materials and Methods
2.1. Greenhouse Studies
2.2. Field Studies
Field Preparation and Treatment Evaluations
2.3. Statistical Analysis
3. Results
3.1. Evaluation of the PIF Approach Under Greenhouse Conditions
3.1.1. Fall Greenhouse Study
3.1.2. Spring Greenhouse Study
3.2. Evaluation of the PIF Approach Under Field Condition
3.2.1. Fall Field Study 1
3.2.2. Spring Field Study
3.2.3. Fall Field Study 2
3.3. Pepper Yield during Two Fall Seasons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osborne, L.S.; Petitt, F.L.; Landa, Z.; Hoelmer, K.A. Biological control of pests attacking crops grown in protected culture: The Florida experience. In Pest Management in the Subtropics: Biological Control—A Florida Perspective; Rosen, D., Bennett, F.D., Capinera, J.L., Eds.; Intercept Ltd.: Andover, UK, 1994; Volume 1, pp. 327–342. [Google Scholar]
- USDA. Census of Agriculture, Census of Horticultural Specialties, Survey 2014. Available online: www.agcensus.usda.gov/Surveys/Census_of_Horticulture_Specialties/index.asp (accessed on 10 April 2018).
- Thomas, M.C. The Exotic Invasion of Florida. A Report on Arthropod Immigration into the Sunshine State; Florida Department of Agriculture and Consumer Services: Tallahassee, FL, USA, 2000; 6p. [Google Scholar]
- Stary, P. Biology of Aphid Parasites (Hymenoptera: Aphidiidae) with Respect to Integrated Control; Series Entomologica 6; Dr. W. Junk: The Hague, The Netherlands, 1970; 641p. [Google Scholar]
- Parr, W.J.; Stacey, D.L. ‘Banker’-plant system of whitefly parasite release on tomatoes. In Report of the Glasshouse Crops Research Institute; Glasshouse Crops Research Institute: Littlehampton, UK, 1976; p. 96. [Google Scholar]
- Bennison, J.A. Biological control of aphids on cucumbers use of open rearing systems or ‘banker plants’ to aid establishment of Aphidius matricariae and Aphidoletes aphidimyza. Meded. Fac. Landbouwwet. Univ. Gent 1992, 57, 457–466. [Google Scholar]
- Luckmann, W.H.; Metcalf, R.L. The pest-management concept. In Introduction to Insect Pest Management; Metcalf, R.L., Luckmann, W.H., Eds.; John Wiley & Sons: New York, NY, USA, 1975; pp. 3–35. [Google Scholar]
- Gonzalez, D.; Wilson, L.T. A food-web approach to economic thresholds: A sequence of pests/predaceous arthropods on California cotton. Entomophaga 1982, 27, 31–43. [Google Scholar] [CrossRef]
- Messelink, G.J.; Maanen, R.; Steenpaal, S.E.F.; Janssen, A. Biological control of thrips and whiteflies by a shared predator: Two pests are better than one. Biol. Control 2008, 44, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Sampson, C. The commercial development of an Amblyseius cucumeris controlled release method for the control of Frankliniella occidentalis in protected crops. In Proceedings of the Brighton Crop Protection Conference: Pests & Diseases, Brighton, UK, 16–19 November 1998; Volume 2, pp. 409–416. [Google Scholar]
- Wade, M.R.; Zalucki, M.P.; Wratten, S.D.; Robinson, K.A. Conservation biological control of arthropods using artificial food sprays: Current status and future challenges. Biol. Control 2008, 45, 185–199. [Google Scholar] [CrossRef]
- Huang, N.; Enkegaard, A.; Osborne, L.S.; Ramakers, P.M.J.; Messelink, G.J.; Pijnakker, J.; Murphy, G. The banker plant method in biological control. Crit. Rev. Plant Sci. 2011, 30, 259–278. [Google Scholar] [CrossRef]
- Ramakers, P.M.J. Manipulation of phytoseiid thrips predators in the absence of thrips. IOBC/WPRS Bull. 1990, 13, 169–172. [Google Scholar]
- Ramakers, P.M.J. Biological control using oligophagous predators. In Thrips Biology and Management; Parker, B.L., Skinner, M., Lewis, T., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 225–229. [Google Scholar]
- McMurtry, J.A.; Croft, B.A. Life-Styles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol. 1997, 42, 291–321. [Google Scholar] [CrossRef]
- Nomikou, M.; Janssen, A.; Sabelis, M.W. Phytoseiid predators of whiteflies feed and reproduce on non-prey food sources. Exp. Appl. Acarol. 2003, 31, 15–26. [Google Scholar] [CrossRef]
- Nomikou, M.; Maurice, W.; Sabelis, M.W.; Janssen, A. Pollen subsidies promote whitefly control through the numerical response of predatory mites. BioControl 2010, 55, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Park, H.H.; Shipp, L.; Buttenhuis, R. Predation, development, and oviposition by the predatory mite Amblyseius swirskii (Acari: Phytoseiidae) on tomato russet mite (Acari: Eriophyidae). J. Econ. Entomol. 2010, 103, 563–569. [Google Scholar] [CrossRef]
- Avery, P.B.; Kumar, V.; Xiao, Y.F.; Powell, C.A.; McKenzie, C.L.; Osborne, L. Selecting an ornamental pepper banker plant for Amblyseius swirskii in floriculture crops. Arthropod Plant Interact. 2014, 8, 49–56. [Google Scholar] [CrossRef]
- Kumar, V.; Wekesa, V.; Avery, P.B.; Powell, C.A.; McKenzie, C.L.; Osborne, L.S. Effect of pollens of various ornamental pepper cultivars on the development and reproduction of Amblyseius swirskii (Acari: Phytoseiidae). Fla. Entomol. 2014, 97, 367–373. [Google Scholar] [CrossRef]
- Calvo, F.J.; Lorente, M.J.; Stansly., P.A.; Belda, J.E. Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol. Exp. Appl. 2012, 143, 111–119. [Google Scholar] [CrossRef]
- Welch, K.D.; Harwood, J.D. Temporal dynamics of natural enemy-pest interactions in a changing environment. Biol. Control 2014, 75, 18–27. [Google Scholar] [CrossRef]
- Gómez-Marco, F.; Tena, A.; Jaques, J.A.; Garcia, A. Early arrival of predators controls Aphis spiraecola colonies in citrus clementines. J. Pest Sci. 2016, 89, 69–79. [Google Scholar] [CrossRef]
- Osborne, L.S.; Barrett, J.E. You can bank on it, banker plants can be used to rear natural enemies to help control greenhouse pests. Ornam. Outlook 2005, 26–27. Available online: https://mrec.ifas.ufl.edu/lso/banker/Documents/BANKERFoliage.pdf (accessed on 21 January 2020).
- Xiao, Y.F.; Avery, P.B.; Chen, J.J.; McKenzie, C.L.; Osborne, L. Ornamental pepper as banker plants for establishment of Amblyseius swirskii (Acari: Phytoseiidae) for biological control of multiple pests in greenhouse vegetable production. Biol. Control 2012, 63, 279–286. [Google Scholar] [CrossRef]
- Kumar, V.; Xiao, Y.; McKenzie, C.L.; Osborne, L.S. Early establishment of the phytoseiid mite Amblyseius swirskii (Acari: Phytoseiidae) on pepper seedlings in a predator-in-first approach. Exp. Appl. Acarol. 2015, 65, 465–481. [Google Scholar] [CrossRef]
- Kutuk, H.; Yigit, A. Pre-establishment of Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae) using Pinus brutia (Ten.) (Pinales: Pinaceae) pollen for thrips (Thysanoptera: Thripidae) control in greenhouse peppers. Int. J. Acarol. 2011, 37, 95–101. [Google Scholar] [CrossRef]
- Kakkar, G.; Kumar, V.; Seal, D.R.; Stansly, P. Predation by Neoseiulus cucumeris and Amblyseius swirskii on Thrips palmi and Frankliniella schultzei on cucumber. Biol. Control 2016, 92, 85–91. [Google Scholar] [CrossRef]
- Butler, D.M.; Kokalis-Burelle, N.; Muramoto, J.; Shennan, C.; McCollum, T.G.; Rosskopf, E.N. Impact of anaerobic soil disinfestation combined with soil solarisation on plant-parasitic nematodes and introduced inoculum of soilborne plant pathogens in raised-bed vegetable production. Crop. Prot. 2012, 39, 33–40. [Google Scholar] [CrossRef]
- Olson, S.M.; Santos, B. Vegetable Production Handbook for Florida; University of Florida–IFAS Extension publication: Gainesville, FL, USA, 2010; Available online: http://edis.ifas.ufl.edu/features/handbooks/vegetableguide.html (accessed on 16 July 2018).
- Kakkar, G.; Seal, D.R.; Kumar, V. Assessing abundance and distribution of an invasive thrips Frankliniella schultzei (Trybom) (Thysanoptera: Thripidae) in South Florida. Bull. Entomol. Res. 2012, 102, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Statistical Analysis Systems (SAS). SAS/STAT®, Release 9.4 User’s Guide; SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
- Van Rijn, P.C.J.; Van Houten, Y.M.; Sabelis, M.W. How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 2002, 83, 2664–2679. [Google Scholar] [CrossRef]
- Ragusa, E.; Tsolakis, H.; Palomero, R.J. Effect of pollens and preys on various biological parameters of the generalist mite Cydnodromus californicus. Bull. Insectol. 2009, 62, 153–158. [Google Scholar]
- Lee, S.H.; Gillespie, D.R. Life tables and development of Amblyseius swirskii (Acari: Phytoseiidae) at different temperatures. Exp. Appl. Acarol. 2011, 53, 17–27. [Google Scholar] [CrossRef] [PubMed]
- USDA. National Agricultural Statistics Service, Quick Stats. 2019. Available online: https://quickstats.nass.usda.gov/ (accessed on 18 July 2019).
- Kakkar, G.; Seal, D.; Stansly, P.; Liburd, O.; Kumar, V. Abundance of Frankliniella schultzei (Thysanoptera: Thripidae) in flowers on major vegetable crops of south Florida. Fla. Entomol. 2012, 97, 468–475. [Google Scholar] [CrossRef]
- Seal, D.R.; Kumar, V.; Kakkar, G.; Mello, S.C. Abundance of adventive Thrips palmi (Thysanoptera: Thripidae) populations in Florida during the first sixteen years. Fla. Entomol. 2013, 96, 789–796. [Google Scholar] [CrossRef]
- Seal, D.R.; Kumar, V.; Kakkar, G. Common blossom thrips, Frankliniella schultzei (Thysanoptera: Thripidae) management and Groundnut ring spot virus prevention on tomato and pepper in Southern Florida. Fla. Entomol. 2014, 97, 374–383. [Google Scholar] [CrossRef]
- Kumar, V.; Kakkar, G.; Seal, D.R.; McKenzie, C.L.; Osborne, L. An overview of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) biology, distribution, and management. In Weed and Pest Control—Conventional and New Challenges; Solenski, S., Larramendy, M., Eds.; Intech: Rijeka, Croatia, 2013; pp. 53–77. [Google Scholar]
- Kumar, V.; Kakkar, G.; Palmer, C.; McKenzie, C.L.; Osborne, L.S. Thrips Management Program for Horticultural Crops; EDIS-online extension publication; EDIS# EENY 987; Entomology and Nematology Department, Florida Department of Plant Industry, Institute of Food and Agricultural Sciences, University of Florida: Florida, FL, USA, 2016; Available online: http://edis.ifas.ufl.edu/in1145 (accessed on 14 December 2019).
- McKenzie, C.L.; Kumar, V.; Palmer, C.L.; Oetting, R.D.; Osborne, L.S. Chemical class rotations for control of Bemisia tabaci (Hemiptera: Aleyrodidae) on poinsettia and their effect on cryptic species population composition. Pest Manag. Sci. 2014, 70, 1573–1587. [Google Scholar] [CrossRef]
- Cluver, J.; Smith, H.; Funderburk, J.; Frantz, G. Western Flower Thrips (Frankliniella occidentalis [Pergande]); ENY 883; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2015; Available online: http://edis.ifas.ufl.edu//IN1089 (accessed on 16 October 2019).











| Fall Season 1 | Fall Season 2 | |||||
|---|---|---|---|---|---|---|
| Treatments | Fruit Count/Plot | Yield/Plot (kg) | Extrapolated Yield (kg/ha) | Fruit Count/Plot | Yield/Plot (kg) | Extrapolated Yield (kg/ha) |
| 7039_0 | 625.3 ± 41.8 ab | 125.2 ± 10.3 ab | 54,659 | 514.0 ± 28.4 c | 112.0 ± 5.4 c | 48,861 |
| 7039_20 | 695.8 ± 35.9 a | 140.3 ± 7.7 a | 61,190 | 604.5 ± 23.4 ab | 131.0 ± 4.8 b | 57,153 |
| 7039_40 | 705.5 ± 34.7 a | 143.7 ± 6.2 a | 62,714 | 628.2 ± 29.9 ab | 140.2 ± 6.4 ab | 61,170 |
| 7039_Sachet | 623.0 ± 32.7 ab | 129.1 ± 6.7 ab | 56,322 | 585.2 ± 20.3 bc | 134.1 ± 6.1 ab | 58,518 |
| 7141_0 | 489.8 ± 51.4 c | 107.9 ± 11.7 b | 47,060 | 406.2 ± 15.9 d | 90.0 ± 3.9 d | 39,283 |
| 7141_20 | 587.2 ± 37.8 b | 128.9 ± 9.7 ab | 56,242 | 619.7 ± 23.2 ab | 139.3 ± 5.1 ab | 60,754 |
| 7141_40 | 644.2 ± 16.0 ab | 142.6 ± 4.4 a | 62,199 | 666.0 ± 41.7 a | 148.9 ± 7.0 a | 64,970 |
| 7141_Sachet | 574.8 ± 29.3 c | 126.2 ± 7.6 ab | 55,075 | 626.7 ± 18.2 ab | 136.2 ± 4.31 ab | 59,409 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Mehra, L.; McKenzie, C.L.; Osborne, L.S. “Predator-In-First”: A Preemptive Biological Control Strategy for Sustainable Management of Pepper Pests in Florida. Sustainability 2020, 12, 7816. https://0-doi-org.brum.beds.ac.uk/10.3390/su12187816
Kumar V, Mehra L, McKenzie CL, Osborne LS. “Predator-In-First”: A Preemptive Biological Control Strategy for Sustainable Management of Pepper Pests in Florida. Sustainability. 2020; 12(18):7816. https://0-doi-org.brum.beds.ac.uk/10.3390/su12187816
Chicago/Turabian StyleKumar, Vivek, Lucky Mehra, Cindy L. McKenzie, and Lance S. Osborne. 2020. "“Predator-In-First”: A Preemptive Biological Control Strategy for Sustainable Management of Pepper Pests in Florida" Sustainability 12, no. 18: 7816. https://0-doi-org.brum.beds.ac.uk/10.3390/su12187816