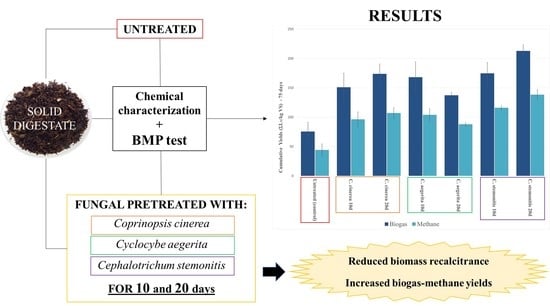
Fungal Pretreatments on Non-Sterile Solid Digestate to Enhance Methane Yield and the Sustainability of Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Sampling and Storage
2.2. Biomass Characterization
2.3. Fungal Pretreatments
2.4. Biochemical Methane Potential Tests
2.5. Statistical Analyses
3. Results
3.1. Fungal Inoculum Addition and Pretreatment of SFD
3.2. BMP Tests
4. Discussion
4.1. SFD Characteristics
4.2. Fungal Inoculum Addition and Pretreatments: Effects on SFD
4.3. BMP Tests
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liguori, R.; Faraco, V. Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour. Technol. 2016, 215, 13–20. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Khanal, S.; Manandhar, A.; Shah, A. Anaerobic digestion for bioenergy production: Global status, environmental and techno-economic implications, and government policies. Bioresour. Technol. 2018, 247, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Fritsche, U.R.; Sims, R.E.H.; Monti, A. Direct and indirect land-use competition issues for energy crops and their sustainable production—An overview. Biofuels Bioprod. Biorefining 2010, 4, 692–704. [Google Scholar] [CrossRef]
- Young, D.; Dollhofer, V.; Callaghan, T.M.; Reitberger, S.; Lebuhn, M.; Benz, J.P. Isolation, identification and characterization of lignocellulolytic aerobic and anaerobic fungi in one- and two-phase biogas plants. Bioresour. Technol. 2018, 268, 470–479. [Google Scholar] [CrossRef]
- Popovic, O.; Gioelli, F.; Dinuccio, E.; Rollè, L.; Balsari, P. Centrifugation of digestate: The effect of chitosan on separation efficiency. Sustainability 2017, 9, 2032. [Google Scholar] [CrossRef] [Green Version]
- Guilayn, F.; Jimenez, J.; Rouez, M.; Crest, M.; Patureau, D. Digestate mechanical separation: Efficiency profiles based on anaerobic digestion feedstock and equipment choice. Bioresour. Technol. 2019, 274, 180–189. [Google Scholar] [CrossRef]
- Dinuccio, E.; Gioelli, F.; Cuk, D.; Rollè, L.; Balsari, P. The use of co-digested solid fraction as feedstock for biogas plants. J. Agric. Eng. 2013, 44, 3–8. [Google Scholar] [CrossRef]
- Sambusiti, C.; Monlau, F.; Ficara, E.; Musatti, A.; Rollini, M.; Barakat, A.; Malpei, F. Comparison of various post-treatments for recovering methane from agricultural digestate. Fuel Process. Technol. 2015, 137, 359–365. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Paavola, T.; Rintala, J. Effects of storage on characteristics and hygienic quality of digestates from four co-digestion concepts of manure and biowaste. Bioresour. Technol. 2008, 99, 7041–7050. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, A.; Cavicchioli, D.; Kremmydas, D.; Rozakis, S.; Olper, A. The impact of different energy policy options on feedstock price and land demand for maize silage: The case of biogas in Lombardy. Energy Policy 2016, 96, 351–363. [Google Scholar] [CrossRef]
- Hansen, T.L.; Sommer, S.G.; Gabriel, S.; Christensen, T.H. Methane Production during Storage of Anaerobically Digested Municipal Organic Waste. J. Environ. Qual. 2006, 35, 830–836. [Google Scholar] [CrossRef] [Green Version]
- Gioelli, F.; Dinuccio, E.; Balsari, P. Residual biogas potential from the storage tanks of non-separated digestate and digested liquid fraction. Bioresour. Technol. 2011, 102, 10248–10251. [Google Scholar] [CrossRef]
- Menardo, S.; Gioelli, F.; Balsari, P. The methane yield of digestate: Effect of organic loading rate, hydraulic retention time, and plant feeding. Bioresour. Technol. 2011, 102, 2348–2351. [Google Scholar] [CrossRef]
- Balsari, P.; Gioelli, F.; Menardo, S.; Paschetta, E. The (re)use of mechanical separated solid fraction of digested or not digested slurry in anaerobic digestion plants. In Proceedings of the 14th Ramiran International Conference, Lisboa, Portugal, 12–15 September 2010; Volume 4630, pp. 2–5. [Google Scholar]
- Zhong, Y.; Liu, Z.; Isaguirre, C.; Liu, Y.; Liao, W. Fungal fermentation on anaerobic digestate for lipid-based biofuel production. Biotechnol. Biofuels 2016, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Rouches, E.; Herpoël-Gimbert, I.; Steyer, J.P.; Carrere, H. Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: A review. Renew. Sustain. Energy Rev. 2016, 59, 179–198. [Google Scholar] [CrossRef]
- Akhtar, N.; Gupta, K.; Goyal, D.; Goyal, A. Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2016, 35, 489–511. [Google Scholar] [CrossRef]
- Merlin Christy, P.; Gopinath, L.R.; Divya, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew. Sustain. Energy Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, Association of Analytical Chemists, 15th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Zhao, J.; Ge, X.; Vasco-Correa, J.; Li, Y. Fungal pretreatment of unsterilized yard trimmings for enhanced methane production by solid-state anaerobic digestion. Bioresour. Technol. 2014, 158, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Nayan, N.; van Erven, G.; Kabel, M.A.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Evaluation of fungal degradation of wheat straw cell wall using different analytical methods from ruminant nutrition perspective. J. Sci. Food Agric. 2019, 99, 4054–4062. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Effectiveness of microbial pretreatment by Ceriporiopsis subvermispora on different biomass feedstocks. Bioresour. Technol. 2011, 102, 7507–7512. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Poulsen, T.G.; Sheng, K. Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl. Energy 2016, 180, 661–671. [Google Scholar] [CrossRef]
- Phutela, U.G.; Sahni, N.; Sooch, S.S. Fungal degradation of paddy straw for enhancing biogas production. Indian J. Sci. Technol. 2011, 4, 660–665. [Google Scholar] [CrossRef]
- Verein Deutscher Ingenieure (VDI) 4630. Fermentation of Organic Materials—Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; VDI Handbuch Energietechnik; Beuth Verlag GmbH: Berlin, Germany, 2006; pp. 44–59. [Google Scholar]
- Yang, Z.; Wang, W.; He, Y.; Zhang, R.; Liu, G. Effect of ammonia on methane production, methanogenesis pathway, microbial community and reactor performance under mesophilic and thermophilic conditions. Renew. Energy 2018, 125, 915–925. [Google Scholar] [CrossRef]
- Musatti, A.; Ficara, E.; Mapelli, C.; Sambusiti, C.; Rollini, M. Use of solid digestate for lignocellulolytic enzymes production through submerged fungal fermentation. J. Environ. Manag. 2017, 199, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vasco-Correa, J.; Ge, X.; Li, Y. Fungal pretreatment of non-sterile miscanthus for enhanced enzymatic hydrolysis. Bioresour. Technol. 2016, 203, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Opatokun, S.A.; Kan, T.; Al Shoaibi, A.; Srinivasakannan, C.; Strezov, V. Characterization of Food Waste and Its Digestate as Feedstock for Thermochemical Processing. Energy Fuels 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Zhi, Z.; Wang, H. White-rot fungal pretreatment of wheat straw with Phanerochaete chrysosporium for biohydrogen production: Simultaneous saccharification and fermentation. Bioprocess Biosyst. Eng. 2014, 37, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Kainthola, J.; Shariq, M.; Kalamdhad, A.S.; Goud, V.V. Enhanced methane potential of rice straw with microwave assisted pretreatment and its kinetic analysis. J. Environ. Manag. 2019, 232, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Menardo, S.; Balsari, P.; Dinuccio, E.; Gioelli, F. Thermal pre-treatment of solid fraction from mechanically-separated raw and digested slurry to increase methane yield. Bioresour. Technol. 2011, 102, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Nuchdang, S.; Vatanyoopaisarn, S.; Phalakornkule, C. Effectiveness of fungal treatment by Coprinopsis cinerea and Polyporus tricholoma on degradation and methane yields of lignocellulosic grass. Int. Biodeterior. Biodegrad. 2015, 104, 38–45. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Valášková, V.; Merhautová, V.; Gabriel, J. Degradation of lignocellulose by Pleurotus ostreatus in the presence of copper, manganese, lead and zinc. Res. Microbiol. 2005, 156, 670–676. [Google Scholar] [CrossRef]
- Liu, S.; Wu, S.; Pang, C.; Li, W.; Dong, R. Microbial pretreatment of corn stovers by solid-state cultivation of Phanerochaete chrysosporium for biogas production. Appl. Biochem. Biotechnol. 2014, 172, 1365–1376. [Google Scholar] [CrossRef]
- Ge, X.; Matsumoto, T.; Keith, L.; Li, Y. Fungal pretreatment of albizia chips for enhanced biogas production by solid-state anaerobic digestion. Energy Fuels 2015, 29, 200–204. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V.; Goel, R. Fungal pretreatment and associated kinetics of rice straw hydrolysis to accelerate methane yield from anaerobic digestion. Bioresour. Technol. 2019, 286, 121368. [Google Scholar] [CrossRef]
- Peterson, R.; Grinyer, J.; Nevalainen, H. Secretome of the coprophilous fungus Doratomyces stemonitis C8, isolated from koala feces. Appl. Environ. Microbiol. 2011, 77, 3793–3801. [Google Scholar] [CrossRef] [Green Version]
- Isikhuemhen, O.S.; Mikiashvili, N.A.; Kelkar, V. Application of solid waste from anaerobic digestion of poultry litter in Agrocybe aegerita cultivation: Mushroom production, lignocellulolytic enzymes activity and substrate utilization. Biodegradation 2009, 20, 351–361. [Google Scholar] [CrossRef]
- López, M.J.; Suárez-Estrella, F.; Vargas-García, M.C.; López-González, J.A.; Verstichel, S.; Debeer, L.; Wierinck, I.; Moreno, J. Biodelignification of agricultural and forest wastes: Effect on anaerobic digestion. Biomass Bioenergy 2013, 58, 343–349. [Google Scholar] [CrossRef]
- Chen, Y.; Sharma-Shivappa, R.R.; Keshwani, D.; Chen, C. Potential of agricultural residues and hay for bioethanol production. Appl. Biochem. Biotechnol. 2007, 142, 276–290. [Google Scholar] [CrossRef]
- Muthangya, M.; Mshandete, A.M.; Kivaisi, A.K. Two-stage fungal pre-treatment for improved biogas production from sisal leaf decortication residues. Int. J. Mol. Sci. 2009, 10, 4805–4815. [Google Scholar] [CrossRef] [Green Version]
- Liew, L.N.; Shi, J.; Li, Y. Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 2012, 46, 125–132. [Google Scholar] [CrossRef]
- Hom-Diaz, A.; Baldi, F.; Blánquez, P.; Lombardi, L.; Martín-González, L.; Vicent, T. Exhausted Fungal Biomass as a Feedstock for Increasing Methane Production During the Anaerobic Digestion of Organic Wastes. Waste Biomass Valorization 2016, 7, 307–315. [Google Scholar] [CrossRef]
- Novel Substrates for Biogas Production: Paxillus involutus and Phaeolus schweinitzii Mushroom Biomass. Available online: https://www.researchgate.net/profile/Janis_Jasko/publication/236844747_Novel_substrates_for_biogas_production_Paxillus_involutus_and_Phaeolus_schweinitzii_mushroom_biomass/links/00b7d5195d073455c6000000.pdf (accessed on 11 October 2020).
- Mutschlechner, M.; Illmer, P.; Wagner, A.O. Biological pre-treatment: Enhancing biogas production using the highly cellulolytic fungus Trichoderma viride. Waste Manag. 2015, 43, 98–107. [Google Scholar] [CrossRef]
- Brémond, U.; Bertrandias, A.; Loisel, D.; Jimenez, J.; Steyer, J.P.; Bernet, N.; Carrere, H. Assessment of fungal and thermo-alkaline post-treatments of solid digestate in a recirculation scheme to increase flexibility in feedstocks supply management of biogas plants. Renew. Energy 2020, 149, 641–651. [Google Scholar] [CrossRef]
- Singh, S.; Harms, H.; Schlosser, D. Screening of ecologically diverse fungi for their potential to pretreat lignocellulosic bioenergy feedstock. Appl. Microbiol. Biotechnol. 2014, 98, 3355–3370. [Google Scholar] [CrossRef]
- Wagner, A.O.; Schwarzenauer, T.; Illmer, P. Improvement of methane generation capacity by aerobic pre-treatment of organic waste with a cellulolytic Trichoderma viride culture. J. Environ. Manag. 2013, 129, 357–360. [Google Scholar] [CrossRef]
- Deng, Y.; Dai, B.; Xu, J.; Liu, X.; Xu, J. Anaerobic co-digestion of rice straw and soybean straw to increase biogas production by pretreatment with Trichoderma reesei RUT C30. Environ. Prog. Sustain. Energy 2018, 37, 1050–1057. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Xia, Y.; Sheng, K. Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour. Technol. 2017, 224, 174–182. [Google Scholar] [CrossRef]
- Reid, I.D. Optimization of solid-state fermentation for selective delignification of aspen wood with Phlebia tremellosa. Enzyme Microb. Technol. 1989, 11, 804–809. [Google Scholar] [CrossRef]


| Untreated SFD (Control) | Fungal-Pretreated SFD | ||||||
|---|---|---|---|---|---|---|---|
| C. cinerea | C. aegerita | C. stemonitis | |||||
| 10d | 20d | 10d | 20d | 10d | 20d | ||
| pH | 9.4 | 8.2 | 8.5 | 8.8 | 9.0 | 9.0 | 9.1 |
| TS [%] | 31.7 | 22.7 | 22.6 | 24.1 | 24.2 | 23.9 | 23.4 |
| Humidity [%] | 68.3 | 77.3 | 77.4 | 75.9 | 75.8 | 76.1 | 76.6 |
| VS [% TS] | 88.1 | 87.4 | 87.7 | 88.7 | 88.2 | 87.8 | 89.1 |
| TN [%] | 0.8 | 0.6 | 0.6 | 0.7 | 0.6 | 0.7 | 0.6 |
| TAN [%] | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| NDF [% TS] | 82.4 | 80.6 | 79.9 | 81.1 | 79.7 | 79.6 | 73.8 |
| ADF [% TS] | 79.7 | 78.4 | 77.8 | 79.3 | 78.1 | 77.7 | 72.7 |
| ADL-Lignin [% TS] | 31.1 | 30.6 | 30.1 | 30.8 | 29.9 | 29.9 | 28.1 |
| Hemicellulose [% TS] | 2.7 | 2.2 | 2.1 | 1.8 | 1.6 | 1.9 | 1.1 |
| Cellulose [% TS] | 48.6 | 47.8 | 47.7 | 48.5 | 48.2 | 47.8 | 44.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanellati, A.; Spina, F.; Rollé, L.; Varese, G.C.; Dinuccio, E. Fungal Pretreatments on Non-Sterile Solid Digestate to Enhance Methane Yield and the Sustainability of Anaerobic Digestion. Sustainability 2020, 12, 8549. https://0-doi-org.brum.beds.ac.uk/10.3390/su12208549
Zanellati A, Spina F, Rollé L, Varese GC, Dinuccio E. Fungal Pretreatments on Non-Sterile Solid Digestate to Enhance Methane Yield and the Sustainability of Anaerobic Digestion. Sustainability. 2020; 12(20):8549. https://0-doi-org.brum.beds.ac.uk/10.3390/su12208549
Chicago/Turabian StyleZanellati, Andrea, Federica Spina, Luca Rollé, Giovanna Cristina Varese, and Elio Dinuccio. 2020. "Fungal Pretreatments on Non-Sterile Solid Digestate to Enhance Methane Yield and the Sustainability of Anaerobic Digestion" Sustainability 12, no. 20: 8549. https://0-doi-org.brum.beds.ac.uk/10.3390/su12208549