Hematite/Graphitic Carbon Nitride Nanofilm for Fenton and Photocatalytic Oxidation of Methylene Blue
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Aqueous Organic Oxidation
3. Results and Discussion
3.1. Structural Analysis
3.2. Methylene Blue Degradation
3.3. Detection of Reactive Species
3.4. Degradation Kinetics
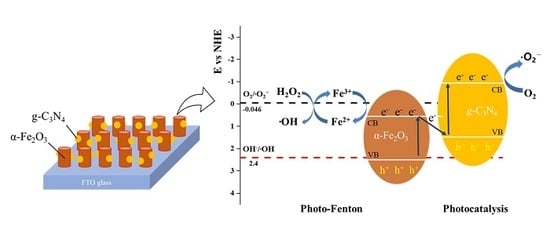
3.5. Methylene Blue Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Rahim Pouran, S.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Pradhan, G.K.; Sahu, N.; Parida, K.M. Fabrication of S, N co-doped α-Fe2O3 nanostructures: Effect of doping, OH radical formation, surface area, [110] plane and particle size on the photocatalytic activity. RSC Adv. 2013, 3, 7912–7920. [Google Scholar] [CrossRef]
- He, L.; Jing, L.; Luan, Y.; Wang, L.; Fu, H. Enhanced Visible Activities of α-Fe2O3 by Coupling N-Doped Graphene and Mechanism Insight. ACS Catal. 2014, 4, 990–998. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z.; Liu, C.S.; Liu, T.X. Effect of alumina on photocatalytic activity of iron oxides for bisphenol A degradation. J. Hazard. Mater. 2007, 149, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, F.; Ni, B.; Xu, J.; Fu, Z.; Lu, Y. Enhanced visible photocatalytic activity of hybrid Pt/α-Fe2O3 nanorods. RSC Adv. 2012, 2, 10057–10063. [Google Scholar] [CrossRef]
- Cao, S.-W.; Fang, J.; Shahjamali, M.M.; Wang, Z.; Yin, Z.; Yang, Y.; Boey, F.Y.C.; Barber, J.; Loo, S.C.J.; Xue, C. In situ growth of Au nanoparticles on Fe2O3 nanocrystals for catalytic applications. CrystEngComm 2012, 14, 7229–7235. [Google Scholar] [CrossRef]
- Kawahara, T.; Yamada, K.; Tada, H. Visible light photocatalytic decomposition of 2-naphthol by anodic-biased α-Fe2O3 film. J. Colloid Interface Sci. 2006, 294, 504–507. [Google Scholar] [CrossRef]
- Tamboli, S.H.; Rahman, G.; Joo, O.-S. Influence of potential, deposition time and annealing temperature on photoelectrochemical properties of electrodeposited iron oxide thin films. J. Alloy. Compd. 2012, 520, 232–237. [Google Scholar] [CrossRef]
- Zhang, Z.; Hossain, M.F.; Takahashi, T. Self-assembled hematite (α-Fe2O3) nanotube arrays for photoelectrocatalytic degradation of azo dye under simulated solar light irradiation. Appl. Catal. B Environ. 2010, 95, 423–429. [Google Scholar] [CrossRef]
- Jaramillo-Páez, C.; Navío, J.A.; Hidalgo, M.C.; Bouziani, A.; Azzouzi, M.E. Mixed α-Fe2O3/Bi2WO6 oxides for photoassisted hetero-Fenton degradation of Methyl Orange and Phenol. J. Photochem. Photobiol. A Chem. 2017, 332, 521–533. [Google Scholar] [CrossRef]
- Sarkar, D.; Singh, A.K. Mechanism of Nonvolatile Resistive Switching in ZnO/α-Fe2O3 Core–Shell Heterojunction Nanorod Arrays. J. Phys. Chem. C 2017, 121, 12953–12958. [Google Scholar] [CrossRef]
- Kang, J.; Kuang, Q.; Xie, Z.-X.; Zheng, L.-S. Fabrication of the SnO2/α-Fe2O3 Hierarchical Heterostructure and Its Enhanced Photocatalytic Property. J. Phys. Chem. C 2011, 115, 7874–7879. [Google Scholar] [CrossRef]
- Guo, L.; Chen, F.; Fan, X.; Cai, W.; Zhang, J. S-doped α-Fe2O3 as a highly active heterogeneous Fenton-like catalyst towards the degradation of acid orange 7 and phenol. Appl. Catal. B Environ. 2010, 96, 162–168. [Google Scholar] [CrossRef]
- Liao, Q.; Sun, J.; Gao, L. Degradation of phenol by heterogeneous Fenton reaction using multi-walled carbon nanotube supported Fe2O3 catalysts. Colloids Surf. A Physicochem. Eng. Asp. 2009, 345, 95–100. [Google Scholar] [CrossRef]
- Chan, J.Y.T.; Ang, S.Y.; Ye, E.Y.; Sullivan, M.; Zhang, J.; Lin, M. Heterogeneous photo-Fenton reaction on hematite (α-Fe2O3){104}, {113} and {001} surface facets. Phys. Chem. Chem. Phys. 2015, 17, 25333–25341. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Li, Z.; Wu, Z.; Zhu, K.; Zhuang, J.; Xi, Q.; Hou, Y.; Chen, J.; Cong, M.; et al. Photo-Fenton reaction and H2O2 enhanced photocatalytic activity of α-Fe2O3 nanoparticles obtained by a simple decomposition route. J. Alloy. Compd. 2019, 771, 398–405. [Google Scholar] [CrossRef]
- Liu, H.; Tong, M.; Zhu, K.; Liu, H.; Chen, R. Preparation and photo-fenton degradation activity of α-Fe2O3 nanorings obtained by adding H2PO4−, SO42−, and citric acid. Chem. Eng. J. 2020, 382, 123010. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, J.; Li, T.; Chen, Y.; Wu, Q.; Xie, T.; Lin, Y.; Dong, S. Visible-light-driven photo-Fenton reaction with α-Fe2O3/BiOI at near neutral pH: Boosted photogenerated charge separation, optimum operating parameters and mechanism insight. J. Colloid Interface Sci. 2019, 554, 531–543. [Google Scholar] [CrossRef]
- Ren, B.; Miao, J.; Xu, Y.; Zhai, Z.; Dong, X.; Wang, S.; Zhang, L.; Liu, Z. A grape-like N-doped carbon/CuO-Fe2O3 nanocomposite as a highly active heterogeneous Fenton-like catalyst in methylene blue degradation. J. Clean. Prod. 2019, 240, 118143. [Google Scholar] [CrossRef]
- Bai, J.; Xu, H.; Chen, G.; Lv, W.; Ni, Z.; Wang, Z.; Yang, J.; Qin, H.; Zheng, Z.; Li, X. Facile fabrication of α-Fe2O3/porous g-C3N4 heterojunction hybrids with enhanced visible-light photocatalytic activity. Mater. Chem. Phys. 2019, 234, 75–80. [Google Scholar] [CrossRef]
- Sun, S.; Ji, C.; Wu, L.; Chi, S.; Qu, R.; Li, Y.; Lu, Y.; Sun, C.; Xue, Z. Facile one-pot construction of α-Fe2O3/g-C3N4 heterojunction for arsenic removal by synchronous visible light catalysis oxidation and adsorption. Mater. Chem. Phys. 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Hao, Q.; Mo, Z.; Chen, Z.; She, X.; Xu, Y.; Song, Y.; Ji, H.; Wu, X.; Yuan, S.; Xu, H.; et al. 0D/2D Fe2O3 quantum dots/g-C3N4 for enhanced visible-light-driven photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2018, 541, 188–194. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Zhang, J.; Lei, J.; Liu, Y. Well-Dispersed Fe2O3 Nanoparticles on g-C3N4 for Efficient and Stable Photo-Fenton Photocatalysis under Visible-Light Irradiation. Eur. J. Inorg. Chem. 2016, 2016, 5387–5392. [Google Scholar] [CrossRef]
- Vayssieres, L.; Beermann, N.; Lindquist, S.-E.; Hagfeldt, A. Controlled Aqueous Chemical Growth of Oriented Three-Dimensional Crystalline Nanorod Arrays: Application to Iron(III) Oxides. Chem. Mater. 2001, 13, 233–235. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, C.; Yuan, G.; Huang, X.; Lü, X.; Cao, Y.; Li, Y.; Hu, A.; Lu, X.; Zhu, P. Morphology-controlled α-Fe2O3 nanostructures on FTO substrates for photoelectrochemical water oxidation. J. Alloy. Compd. 2017, 715, 230–236. [Google Scholar] [CrossRef]
- Raja, K.; Mary Jaculine, M.; Jose, M.; Verma, S.; Prince, A.A.M.; Ilangovan, K.; Sethusankar, K.; Jerome Das, S. Sol–gel synthesis and characterization of α-Fe2O3 nanoparticles. Superlattices Microstruct. 2015, 86, 306–312. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Qiao, F.; Wang, J.; Ai, S.; Li, L. As a new peroxidase mimetics: The synthesis of selenium doped graphitic carbon nitride nanosheets and applications on colorimetric detection of H2O2 and xanthine. Sens. Actuators B Chem. 2015, 216, 418–427. [Google Scholar] [CrossRef]
- Su, S.; Liu, Y.; Liu, X.; Jin, W.; Zhao, Y. Transformation pathway and degradation mechanism of methylene blue through β-FeOOH@GO catalyzed photo-Fenton-like system. Chemosphere 2019, 218, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Abou-Gamra, Z.M.; Ahmed, M.A. Synthesis of mesoporous TiO2–curcumin nanoparticles for photocatalytic degradation of methylene blue dye. J. Photochem. Photobiol. B Biol. 2016, 160, 134–141. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Pal Chaudhary, R. Photocatalytic degradation of methylene blue with Cu doped ZnS nanoparticles. J. Lumin. 2014, 145, 6–12. [Google Scholar] [CrossRef]
- Jing, H.-P.; Wang, C.-C.; Zhang, Y.-W.; Wang, P.; Li, R. Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv. 2014, 4, 54454–54462. [Google Scholar] [CrossRef]
- Atout, H.; Álvarez, M.G.; Chebli, D.; Bouguettoucha, A.; Tichit, D.; Llorca, J.; Medina, F. Enhanced photocatalytic degradation of methylene blue: Preparation of TiO2/reduced graphene oxide nanocomposites by direct sol-gel and hydrothermal methods. Mater. Res. Bull. 2017, 95, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Saleh, T.A.; Pathania, D.; Rathore, B.S.; Sharma, G. A cellulose acetate based nanocomposite for photocatalytic degradation of methylene blue dye under solar light. Ionics 2015, 21, 1787–1793. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, H.; Wang, W.; Zhang, L. TiO2/graphene porous composite and its photocatalytic degradation of methylene blue. Mater. Des. 2016, 108, 632–639. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, C.; Zhang, Y.; Gong, Y.; Wu, J.; Fu, Q.; Pan, C. Construction of hierarchical TiO2 nanorod array/graphene/ZnO nanocomposites for high-performance photocatalysis. J. Mater. Sci. 2018, 53, 15376–15389. [Google Scholar] [CrossRef]
- Niu, X.; Yan, W.; Shao, C.; Zhao, H.; Yang, J. Hydrothermal synthesis of Mo-C co-doped TiO2 and coupled with fluorine-doped tin oxide (FTO) for high-efficiency photodegradation of methylene blue and tetracycline: Effect of donor-acceptor passivated co-doping. Appl. Surf. Sci. 2019, 466, 882–892. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Liu, W.; Zhang, S. Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4-RGO-TiO2 nanoheterojunctions. Appl. Surf. Sci. 2017, 405, 60–70. [Google Scholar] [CrossRef]
- Ma, D.; Wu, J.; Gao, M.; Xin, Y.; Sun, Y.; Ma, T. Hydrothermal synthesis of an artificial Z-scheme visible light photocatalytic system using reduced graphene oxide as the electron mediator. Chem. Eng. J. 2017, 313, 1567–1576. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.-T.F.; et al. High Efficiency Photocatalytic Water Splitting Using 2D α-Fe2O3/g-C3N4 Z-Scheme Catalysts. Adv. Energy Mater. 2017, 7, 1700025. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, B.; Jiang, C.; Cheng, B.; Yu, J. Constructing 2D/2D Fe2O3/g-C3N4 Direct Z-Scheme Photocatalysts with Enhanced H2 Generation Performance. Sol. RrL 2018, 2, 1800006. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A Hierarchical Z-Scheme α-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Ibrahim, S.S.; Shahien, M.G. Photocatalytic degradation and photo-Fenton oxidation of Congo red dye pollutants in water using natural chromite—Response surface optimization. Appl. Water Sci. 2017, 7, 4743–4756. [Google Scholar] [CrossRef] [Green Version]










| Type of Oxidation Process | Ka (min−1) | R2 |
|---|---|---|
| FTO + UV | 1.40 × 10−4 | 0.99 |
| α-Fe2O3 + UV | 1.80 × 10−3 | 0.99 |
| α-Fe2O3/g-C3N4 + UV | 5.65 × 10−3 | 0.99 |
| FTO + UV + H2O2 | 4.11 × 10−3 | 0.99 |
| α-Fe2O3 + UV + H2O2 | 1.52 × 10−2 | 0.99 |
| α-Fe2O3/g-C3N4 + UV + H2O2 | 3.67 × 10−2 | 0.99 |
| H2O2 (mM) | pH | Ka (min−1) | R2 |
|---|---|---|---|
| 0.1 | 7 | 1.10 × 10−2 | 0.99 |
| 0.2 | 7 | 1.50 × 10−2 | 0.99 |
| 0.5 | 7 | 2.73 × 10−2 | 0.99 |
| 1 | 7 | 3.67 × 10−2 | 0.99 |
| 2 | 7 | 3.72 × 10−2 | 0.99 |
| 1 | 3 | 6.13 × 10−2 | 0.99 |
| 1 | 5 | 5.15 × 10−2 | 0.99 |
| 1 | 9 | 1.07 × 10−2 | 0.99 |
| 1 | 11 | 4.70 × 10−3 | 0.99 |
| Catalyst | Ka (/min) | Light Source | Reference |
|---|---|---|---|
| TiO2–curcumin | 3.05 × 10−2 | UV | [34] |
| Cu doped ZnS | 1.45 × 10−2 | UV | [35] |
| ZIF-8 | 1.70 × 10−2 | UV | [36] |
| TiO2/rGO nanocomposites | 1.12 × 10−2 | UV | [37] |
| CA/TPNC | 1.26 × 10−2 | Solar | [38] |
| TiO2/graphene | 2.08 × 10−2 | Solar | [39] |
| TiO2 NRAs/graphene/ZnO NPs | 1.65 × 10−2 | Visible | [40] |
| (Mo,C)-TiO2/FTO | 2.95 × 10−2 | Visible | [41] |
| g-C3N4-10RGO-TiO2 | 1.47 × 10−2 | Visible | [42] |
| This study (1mM H2O2, pH 7) | 3.67 × 10−2 | UV | - |
| This study (1mM H2O2, pH 3) | 6.13 × 10−2 | UV | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Park, J.-W. Hematite/Graphitic Carbon Nitride Nanofilm for Fenton and Photocatalytic Oxidation of Methylene Blue. Sustainability 2020, 12, 2866. https://0-doi-org.brum.beds.ac.uk/10.3390/su12072866
Lee S, Park J-W. Hematite/Graphitic Carbon Nitride Nanofilm for Fenton and Photocatalytic Oxidation of Methylene Blue. Sustainability. 2020; 12(7):2866. https://0-doi-org.brum.beds.ac.uk/10.3390/su12072866
Chicago/Turabian StyleLee, Sangbin, and Jae-Woo Park. 2020. "Hematite/Graphitic Carbon Nitride Nanofilm for Fenton and Photocatalytic Oxidation of Methylene Blue" Sustainability 12, no. 7: 2866. https://0-doi-org.brum.beds.ac.uk/10.3390/su12072866