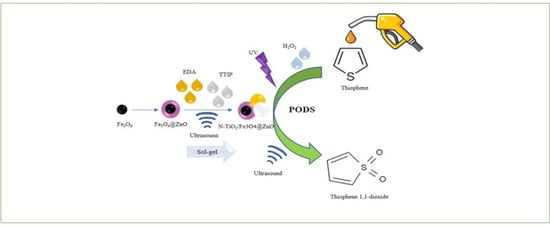
Ultrasound-Assisted Synthesis of a N-TiO2/Fe3O4@ZnO Complex and Its Catalytic Application for Desulfurization
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Magnetic Fe3O4 Nanoparticles
2.3. Synthesis of Fe3O4@ZnO ‘Core-Shell’ Nanoparticles
2.4. Synthesis of N-Doped TiO2 Supported on Magnetic NPs
2.5. Characterization of the Synthesized Catalyst
2.5.1. Particle Size Analysis
2.5.2. X-ray Diffraction Analysis
2.5.3. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.6. Studies of Desulfurization of Thiophene
2.6.1. Experimental Methodology
2.6.2. Analysis Using HPLC
3. Results and Discussions
3.1. Effects of US Parameters on Particle Size
3.1.1. Effect of US Power
3.1.2. Effect of US Irradiation Time
3.1.3. Comparison with Particle Size of the Conventionally Synthesized Catalyst
3.2. Characterization of the Synthesized Catalyst
3.2.1. XRD Analysis
3.2.2. FTIR Analysis
3.3. Catalytic Activity of the Synthesized Catalyst for Desulfurization
3.3.1. Comparative Study of the Activity of Ultrasonically and Conventionally Synthesized Catalysts
3.3.2. Comparative Study of the Activity of Catalysts with and without ZnO in the Support Matrix
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Mominou, N.; Wang, Z.; Liu, L.; Wang, L. Ultra-deep Desulfurization of Gasoline with CuW/TiO2–GO through Photocatalytic Oxidation. Energy Fuel 2016, 30, 962–967. [Google Scholar] [CrossRef]
- Martín, J.M.C.; Sánchez, M.D.C.C.; Presas, P.P.; Fierro, J.L.G. Oxidative processes of desulfurization of liquid fuels. J. Chem. Technol. Biotechnol. 2010, 85, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Saleh, T.A.; Sulaiman, K.O.; Al-Hammadi, S.A.; Dafalla, H.; Danmaliki, G.I. Adsorptive desulfurization of thiophene, benzothiophene and dibenzothiophene over activated carbon manganese oxide nanocomposite: With column system evaluation. J. Clean. Prod. 2017, 154, 401–412. [Google Scholar] [CrossRef]
- Keynejad, K.; Nikazar, M.; Dabir, B. Diesel desulfurization using a UV-photocatalytic process. Pet. Sci. Technol. 2017, 35, 813–819. [Google Scholar] [CrossRef]
- Dedual, G.; MacDonald, M.J.; Alshareef, A.; Wu, Z.; Tsang, D.; Yip, A. Requirements for effective photocatalytic oxidative desulfurization of a thiophene-containing solution using TiO2. J. Environ. Chem. Eng. 2014, 2, 1947–1955. [Google Scholar] [CrossRef]
- Srivastava, V.C. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Samokhvalov, A. Desulfurization of Real and Model Liquid Fuels Using Light: Photocatalysis and Photochemistry. Catal. Rev. 2012, 54, 281–343. [Google Scholar] [CrossRef]
- Yengejeh, S.M.; Allahyari, S.; Rahei, N. Efficient oxidative desulfurization of model fuel by visible-light-driven MoS2-CeO2/SiO2-Al2O3 nano photocatalyst coating. Process Saf. Environ. Prot. 2020, 143, 25–35. [Google Scholar] [CrossRef]
- Abbas, M.N.; Ibrahim, S.A. Catalytic and thermal desulfurization of light naphtha fraction. J. King Saud Univ.—Eng. Sci. 2020, 32, 229–235. [Google Scholar] [CrossRef]
- Yu, G.; Lu, S.; Chen, A.H.; Zhu, Z. Oxidative Desulfurization of Diesel Fuels with Hydrogen Peroxide in the Presence of Activated Carbon and Formic Acid. Energy Fuels 2004, 19, 447–452. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Chen, F.; Chen, Z.; Qian, J.; Zhang, Q. Biotemplating synthesis of N-doped two-dimensional CeO2–TiO2 nanosheets with enhanced visible light photocatalytic desulfurization performance. J. Alloys Compd. 2020, 815, 152326. [Google Scholar] [CrossRef]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Synthesis and characterization of N-doped TiO2 nanoparticles and their application in photocatalytic oxidation of dibenzothiophene under visible light. Ceram. Int. 2016, 42, 14834–14842. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, T.; Liu, H.; Gao, X.; Wang, C.; Wang, G. Desulfurization through Photocatalytic Oxidation: A Critical Review. ChemSusChem 2021, 14, 492–511. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Kait, C.F.; Mutalib, M.I.A. Photooxidative desulfurization for diesel using Fe/N− TiO2 photocatalyst. AIP Conf. Proc. Am. Inst. Phys. 2014, 1621, 10–16. [Google Scholar]
- Feng, Z.; Zhu, Y.; Zhou, Q.; Wu, Y.; Wu, T. Magnetic WO3/Fe3O4 as catalyst for deep oxidative desulfurization of model oil. Mater. Sci. Eng. B 2019, 240, 85–91. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Nemati, A.; Haider, W.; Ghanbarnezhad, S.; Rahman, Z.U.; Ahmed, S.N. Synthesis and Characterization of Nanocomposite of Functionalized Graphene Oxide with Multi Core-Shell Fe3O4-ZnO-TiO2 Nanoparticles. Int. Conf.-Oretical Appl. Nanosci. Nanotechnol. 2017, 111, 1–7. [Google Scholar]
- Abdul-Kadhim, W.; Deraman, M.A.; Abdullah, S.B.; Tajuddin, S.N.; Yusoff, M.M.; Taufiq-Yap, Y.H.; Rahim, M.H.A. Efficient and reusable iron-zinc oxide catalyst for oxidative desulfurization of model fuel. J. Environ. Chem. Eng. 2017, 5, 1645–1656. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarnezhad, S.; Baghshahi, S.; Nemati, A.; Mahmoodi, M. Preparation, magnetic properties, and photocatalytic performance under natural daylight irradiation of Fe3O4-ZnO core/shell nanoparticles designed on reduced GO platelet. Mater. Sci. Semicond. Process. 2017, 72, 85–92. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, A.; Faghihian, H.; Sanati, A.M. Elimination of dibenzothiophene from transportation fuel by combined photocatalytic and adsorptive method. Mater. Sci. Semicond. Process. 2018, 87, 110–118. [Google Scholar] [CrossRef]
- Gogate, P.R. Improvements in Catalyst Synthesis and Photocatalytic Oxidation Processing Based on the Use of Ultrasound. In Heterogeneous Photocatalysis. Topics in Current Chemistry Collections; Muñoz-Batista, M., Navarrete Muñoz, A., Luque, R., Eds.; Springer: Cham, Switzerland, 2020; Volume 378, pp. 71–105. [Google Scholar]
- Wu, Z.; Ondruschka, B. Ultrasound-assisted oxidative desulfurization of liquid fuels and its industrial application. Ultrason. Sonochem. 2010, 17, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Sutkar, V.S.; Gogate, P.R. Design aspects of sonochemical reactors: Techniques for understanding cavitational activity distribution and effect of operating parameters. Chem. Eng. J. 2009, 155, 26–36. [Google Scholar] [CrossRef]
- Ammar, S.H.; Abdulnabi, W.A.; Kader, H.D.A. Synthesis, characterization and environmental remediation applications of polyoxometalates-based magnetic zinc oxide nanocomposites (Fe3O4@ZnO/PMOs). Environ. Nanotechnol. Monit. Manag. 2020, 13, 100289. [Google Scholar] [CrossRef]
- Lei, Y.; Ding, J.; Yu, P.; He, G.; Chen, Y.; Chen, H. Low-temperature preparation of magnetically separable Fe3O4@ZnO-RGO for high-performance removal of methylene blue in visible light. J. Alloys Compd. 2020, 821, 153366. [Google Scholar] [CrossRef]
- Zhou, F.; Song, H.; Wang, H.; Komarneni, S.; Yan, C. N-doped TiO2/sepiolite nanocomposites with enhanced visible-light catalysis: Role of N precursors. Appl. Clay Sci. 2018, 166, 9–17. [Google Scholar] [CrossRef]
- Tiple, A.; Sinhmar, P.S.; Gogate, P.R. Improved direct synthesis of TiO2 catalyst using sonication and its application for the desulfurization of thiophene. Ultrason. Sonochem. 2021, 73, 105547. [Google Scholar] [CrossRef]
- Prasad, K.; Pinjari, D.; Pandit, A.; Mhaske, S. Synthesis of titanium dioxide by ultrasound assisted sol–gel technique: Effect of amplitude (power density) variation. Ultrason. Sonochem. 2010, 17, 697–703. [Google Scholar] [CrossRef]
- Yang, G.; Lin, W.; Lai, H.; Tong, J.; Lei, J.; Yuan, M.; Zhang, Y.; Cui, C. Understanding the relationship between particle size and ultrasonic treatment during the synthesis of metal nanoparticles. Ultrason. Sonochem. 2021, 73, 105497. [Google Scholar] [CrossRef]
- Shirsath, S.; Pinjari, D.; Gogate, P.; Sonawane, S.; Pandit, A. Ultrasound assisted synthesis of doped TiO2 nano-particles: Characterization and comparison of effectiveness for photocatalytic oxidation of dyestuff effluent. Ultrason. Sonochem. 2013, 20, 277–286. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, Y. Ultrasound Assisted Synthesis of Size-Controlled Aqueous Colloids for the Fabrication of Nanoporous Zirconia Membrane. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahendran, V.; Gogate, P.R. Degradation of Acid Scarlet 3R dye using oxidation strategies involving photocatalysis based on Fe doped TiO2 photocatalyst, ultrasound and hydrogen peroxide. Sep. Purif. Technol. 2021, 274, 119011. [Google Scholar] [CrossRef]
- Jordens, J.; Appermont, T.; Gielen, B.; Van Gerven, T.; Braeken, L. Sonofragmentation: Effect of Ultrasound Frequency and Power on Particle Breakage. Cryst. Growth Des. 2016, 16, 6167–6177. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Kusters, K.A.; Pratsinis, S.E.; Thoma, S.G.; Smith, D.M. Ultrasonic fragmentation of agglomerate powders. Chem. Eng. Sci. 1993, 48, 4119–4127. [Google Scholar] [CrossRef]
- Beltran-Huarac, J.C.; Singh, S.P.; Tomar, M.S.; Peňa, S.; Rivera, L.; Perales-Perez, O.J. Synthesis of Fe3O4/ZnO Core-shell Nanoparticles for Photodynamic Therapy Applications. Mater. Res. Soc. Proc. 2010, 1257, 1257-O06-04. [Google Scholar] [CrossRef]
- Wang, X.-K.; Wang, C.; Guo, W.-L.; Wang, J.-G. A novel single-step synthesis of N-doped TiO2 via a sonochemical method. Mater. Res. Bull. 2011, 46, 2041–2044. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Chen, X. Preparation of N-doped visible-light response nanosize TiO2 photocatalyst using the acid-catalyzed hydrolysis method. Chin. J. Catal. 2006, 27, 697–702. [Google Scholar] [CrossRef]
- Li, Y.; Xie, C.; Peng, S.; Lu, G.; Li, S. Eosin Y-sensitized nitrogen-doped TiO2 for efficient visible light photocatalytic hydrogen evolution. J. Mol. Catal. A Chem. 2008, 282, 117–123. [Google Scholar] [CrossRef]
- Wang, D.; Liu, N.; Zhang, J.; Zhao, X.; Zhang, W.; Zhang, M. Oxidative desulfurization using ordered mesoporous silicas as catalysts. J. Mol. Catal. A Chem. 2014, 393, 47–55. [Google Scholar] [CrossRef]
- Amiri, O.; Beshkar, S.; Ahmed, S.S.; Hamad, B.W.; Mahmood, P.H.; Dezaye, A.A. Magnetically-driven Ag/Fe3O4/graphene ternary nanocomposite as efficient photocatalyst for desulfurization of thiophene under visible-light irradiation. Int. J. Hydrogen Energy 2021, 46, 19913–19925. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Evaluating the efficiency of nano-sized Cu doped TiO2/ZnO photocatalyst under visible light irradiation. J. Mol. Liq. 2018, 258, 354–365. [Google Scholar] [CrossRef]
- Fatimah, I. Novitasari Preparation of TiO2-ZnO and its activity test in sonophotocatalytic degradation of phenol. IOP Conf. Series: Mater. Sci. Eng. 2016, 107, 012003. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, Z.; Tang, A.; Zhang, C.; Chen, Z.; Liu, T.; Dong, B. Photocatalytic oxidation of thiophene om RuO2/ SO42−: Insights for cocatalyst and solid-acid. Appl. Catal. B Environ. 2016, 188, 253–258. [Google Scholar] [CrossRef]
- Aazam, E.S. Visible light photocatalytic degradation of thiophene using Ag–TiO2/multi-walled carbon nanotubes nanocomposite. Ceram. Int. 2014, 40, 6705–6711. [Google Scholar] [CrossRef]
- Guo, G.; Guo, H.; Wang, F.; France, L.J.; Yang, W.; Mei, Z.; Yu, Y. Dye-sensitized TiO2@SAB-15 composites: Preparation and their application in photocatalytic desulfurization. Green Energy Environ. 2020, 5, 114–120. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, M.; Tian, M.; Zhao, W. In situ hydrothermal preparation and photocatalytic desulfurization performance of graphene wrapped TiO2 composites. J. Solid State Chem. 2019, 279, 120953. [Google Scholar] [CrossRef]










| Sr. No. | Power | Mean Size (μm) | D10 (μm) | D50 (μm) | D90 (μm) |
|---|---|---|---|---|---|
| 1 | 80 | 31.22 | 7.078 | 25.73 | 63.37 |
| 2 | 90 | 52.91 | 9.134 | 37.73 | 121.8 |
| 3 | 100 | 52.97 | 11.84 | 42.2 | 111.1 |
| 4 | 110 | 51.89 | 6.388 | 32.51 | 129.7 |
| 5 | 120 | 304.4 | 13.42 | 85.53 | 1036 |
| Sr. No. | Time (min) | Mean Size (μm) | D10 (μm) | D50 (μm) | D90 (μm) |
|---|---|---|---|---|---|
| 1 | 15 | 38.67 | 0.063 | 5.268 | 135.2 |
| 2 | 30 | 31.22 | 7.078 | 25.73 | 63.37 |
| 3 | 45 | 40.47 | 0.068 | 21.59 | 112.5 |
| 4 | 60 | 324.5 | 0.053 | 73.86 | 1101 |
| 5 | 75 | 78.73 | 0.051 | 34.74 | 228 |
| Sr. No. | Sample | Mean Size (μm) | D10 (μm) | D50 (μm) | D90 (μm) |
|---|---|---|---|---|---|
| 1 | Conventional | 806.4 | 403.9 | 791.3 | 1229 |
| Sr. No. | TiO2 Complex | Target Compound | Conversion (%) | Time (h) | Photo-Irradiation | Reference |
|---|---|---|---|---|---|---|
| 1 | TiO2-P25 | Thiophene | 91 | 3 | UV | [5] |
| 2 | CuW/TiO2−GO | FCC gasoline model oil | 100 | 1 | UV | [1] |
| 3 | RuO2/SO4 2−-TiO2 | Thiophene | 88 | 3 | UV | [45] |
| 4 | TiO2 powder | Diesel sample | 97 | 0.83 | UV | [4] |
| 5 | Ag-TiO2/MWCNTs | Thiophene | 100 | 0.5 | Visible light | [46] |
| 6 | N-doped TiO2 | Dibenzothiophene | 40.3 | 4 | Visible light | [12] |
| 7 | Dye-sensitized TiO2@SBA-15 | Dibenzothiophene Benzothiophene Thiophene | 96.1 87.5 79 | 1.5 | Visible light | [47] |
| 8 | N-doped CeO2-TiO2 nanosheets | Dibenzothiophene | 93.7 | 3 | Visible light | [11] |
| 9 | RGO/TiO2 | Thiophene | 94.3 | 1.67 | Visible light | [48] |
| 10 | N-TiO2/Fe3O4@ZnO | Thiophene | 47 | 1.67 | UV | Current work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalvi, P.; Dey, A.; Gogate, P.R. Ultrasound-Assisted Synthesis of a N-TiO2/Fe3O4@ZnO Complex and Its Catalytic Application for Desulfurization. Sustainability 2022, 14, 16201. https://0-doi-org.brum.beds.ac.uk/10.3390/su142316201
Dalvi P, Dey A, Gogate PR. Ultrasound-Assisted Synthesis of a N-TiO2/Fe3O4@ZnO Complex and Its Catalytic Application for Desulfurization. Sustainability. 2022; 14(23):16201. https://0-doi-org.brum.beds.ac.uk/10.3390/su142316201
Chicago/Turabian StyleDalvi, Payal, Ananya Dey, and Parag R. Gogate. 2022. "Ultrasound-Assisted Synthesis of a N-TiO2/Fe3O4@ZnO Complex and Its Catalytic Application for Desulfurization" Sustainability 14, no. 23: 16201. https://0-doi-org.brum.beds.ac.uk/10.3390/su142316201