Pharmacokinetics of Toxin-Derived Peptide Drugs
Abstract
:1. Introduction
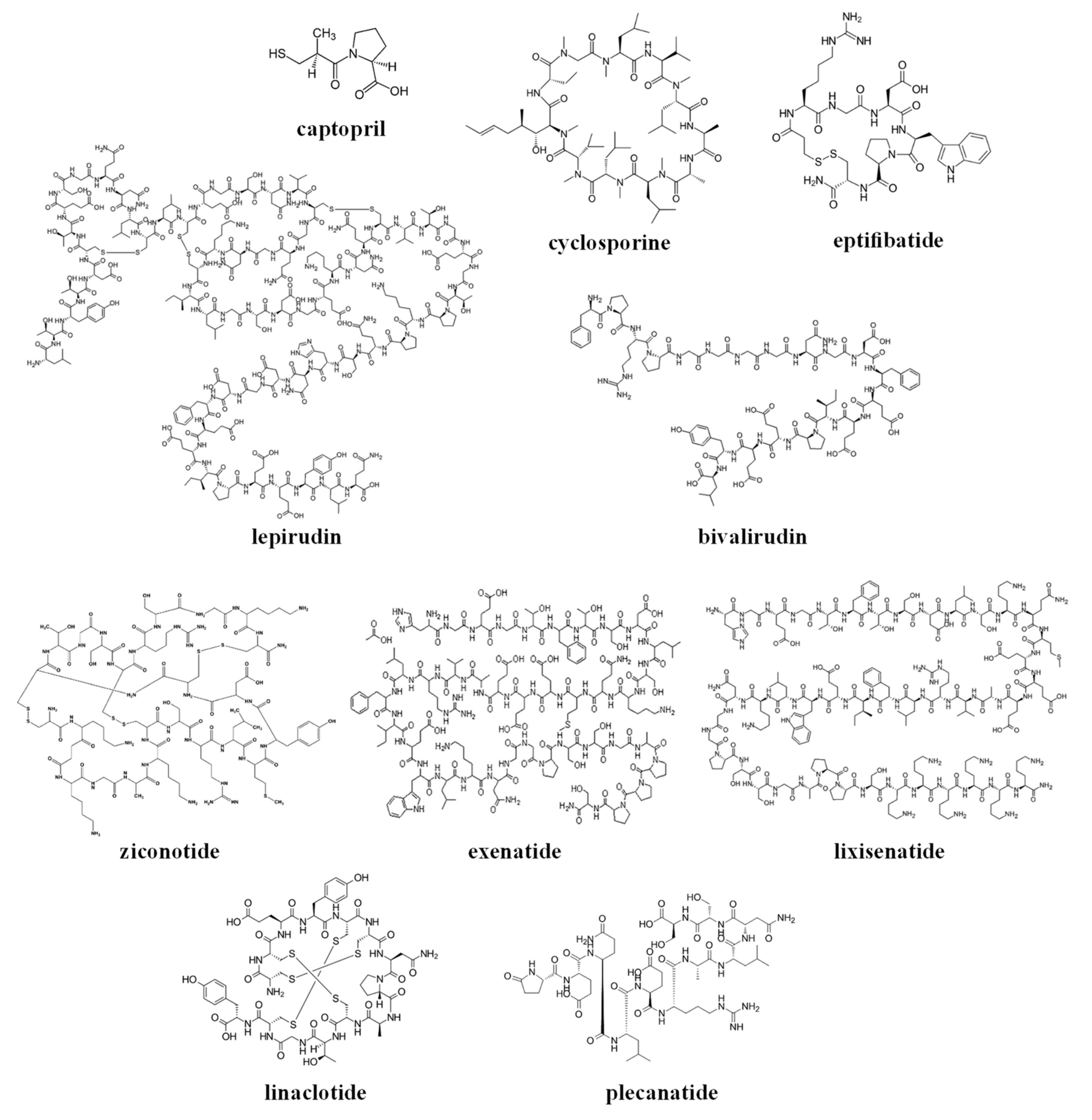
2. Structure and Physicochemical Properties of Clinically-Approved Toxin-Derived Peptide Drugs
3. Pharmacokinetic Properties of the Clinically Approved Toxin-Derived Peptide Drugs
3.1. Captopril
3.2. Cyclosporine
3.3. Eptifibatide
3.4. Lepirudin
3.5. Bivalirudin
3.6. Ziconotide
3.7. Exenatide (IR and SR Formulations)
3.8. Lixisenatide
3.9. Linaclotide
3.10. Plecanatide
4. Concluding Remarks and Perspectives
Funding
Conflicts of Interest
References
- Norton, R.S. Enhancing the therapeutic potential of peptide toxins. Expert Opin. Drug Discov. 2017, 12, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Czerwinski, A.; Norton, R.S. Peptide therapeutics from venom: Current status and potential. Bioorg. Med. Chem. 2018, 26, 2738–2758. [Google Scholar] [CrossRef] [PubMed]
- Provisional BCS Classification Database. Available online: http://www.ddfint.net/search.cfm (accessed on 19 October 2018).
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Captopril Tablets, USP. Professional Prescribing Information; Par Pharmaceutical Companies, Inc.: Woodcliff Lake, NJ, USA, 2012. [Google Scholar]
- Duchin, K.L.; McKinstry, D.N.; Cohen, A.I.; Migdalof, B.H. Pharmacokinetics of captopril in healthy subjects and in patients with cardiovascular diseases. Clin. Pharmacokinet. 1988, 14, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Vertes, V.; Haynie, R. Comparative pharmacokinetics of captopril, enalapril, and quinapril. Am. J. Cardiol. 1992, 69, 8C–16C. [Google Scholar] [CrossRef]
- Richer, C.; Giroux, B.; Plouin, P.F.; Maarek, B.; Giudicelli, J.F. Captopril: Pharmacokinetics, antihypertensive and biological effects in hypertensive patients. Br. J. Clin. Pharmacol. 1984, 17, 243–250. [Google Scholar] [CrossRef] [PubMed]
- NEORAL® Soft Gelatin Capsules (cyclosporine capsules, USP) modified. Professional Prescribing Information; Novartis Pharmaceuticals Corporation: East Hanover, NJ, USA, 2001. [Google Scholar]
- Masuda, S.; Inui, K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol. Ther. 2006, 112, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Pillai, V.C.; Venkataramanan, R. Population pharmacokinetics of cyclosporine in transplant recipients. AAPS J. 2013, 15, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.H.; Cui, Y.M.; Guo, J.F.; Zhou, Y.; Zhai, S.D.; Cui, F.D.; Lu, W. Population pharmacokinetics of cyclosporine in clinical renal transplant patients. Drug Metab. Dispos. 2005, 33, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, D.A.; van Gelder, T.; van Schaik, R.H.; Balk, A.H.; van der Heiden, I.P.; van Dam, T.; van der Werf, M.; Weimar, W.; Mathot, R.A. Population pharmacokinetics of cyclosporine in kidney and heart transplant recipients and the influence of ethnicity and genetic polymorphisms in the MDR-1, CYP3A4, and CYP3A5 genes. Clin. Pharmacol. Ther. 2004, 76, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Kim, I.W.; Choi, B.; Han, N.; Yun, H.Y.; Park, S.; Oh, J.M. Population pharmacokinetics of cyclosporine in hematopoietic stem cell transplant patients: Consideration of genetic polymorphisms. Ann. Pharmacother. 2015, 49, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Integrilin™ (Eptifibatide) Injection. Professional Prescribing Information; Key Pharmaceuticals, Inc.: Kenilworth, NJ, USA, 1998. [Google Scholar]
- American Society of Health-System Pharmacists. Drug Information 2015; Pharmaceutical Press: Bethesda, MD, USA, 2015; p. 1541. [Google Scholar]
- Smith, B.S.; Gandhi, P.J. Pharmacokinetics and pharmacodynamics of low-molecular-weight heparins and glycoprotein IIb/IIIa receptor antagonists in renal failure. J. Thromb. Thrombolysis 2001, 11, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gretler, D.D.; Guerciolini, R.; Williams, P.J. Pharmacokinetic and pharmacodynamic properties of eptifibatide in subjects with normal or impaired renal function. Clin. Ther. 2004, 26, 390–398. [Google Scholar] [CrossRef]
- Refludan (Lepirudin) Injection. Professional Prescribing Information; TZLB Behring GmbH: Marburg, Germany, 2004. [Google Scholar]
- Greinacher, A. Lepirudin: A bivalent direct thrombin inhibitor for anticoagulation therapy. Expert Rev. Cardiovasc. Therapy 2004, 2, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Seidel, H.; Kolde, H.-J. Monitoring of Argatroban and Lepirudin: What is the Input of Laboratory Values in “Real Life”? Clin. Appl. Thromb. Hemost. 2018, 24, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Wittkowsky, A.K.; Kondo, L.M. Lepirudin dosing in dialysis-dependent renal failure. Pharmacotherapy 2000, 20, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Angiomax (Bivalirudin) Injection. In Professional Prescribing Information; The Medicines Company: Cambridge, MA, USA, 2016.
- Shammas, N.W. Bivalirudin: Pharmacology and clinical applications. Cardiovasc. Drug Rev. 2005, 23, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Van De Car, D.A.; Rao, S.V.; Ohman, E.M. Bivalirudin: A review of the pharmacology and clinical application. Expert Rev. Cardiovasc. Ther. 2010, 8, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Wang, K.; Zhao, X.; Li, Y.F.; Zheng, Q.S.; Wang, Z.N.; Cui, Y.M. Population pharmacokinetics and pharmacodynamics of bivalirudin in young healthy Chinese volunteers. Acta Pharmacol. Sin. 2012, 33, 1387–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robson, R.; White, H.; Aylward, P.; Frampton, C. Bivalirudin pharmacokinetics and pharmacodynamics: Effect of renal function, dose, and gender. Clin. Pharmacol. Ther. 2002, 71, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.E.; Deer, T.R. Ziconotide: A clinical update and pharmacologic review. Expert Opin. Pharmacother. 2013, 14, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Prialt (Ziconotide Intrathecal Infusion). Professional Prescribing Information; Elan Pharmaceuticals, Inc.: San Diego, CA, USA, 2004. [Google Scholar]
- McGivern, J.G. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Wermeling, D.; Drass, M.; Ellis, D.; Mayo, M.; McGuire, D.; O’Connell, D.; Hale, V.; Chao, S. Pharmacokinetics and pharmacodynamics of intrathecal ziconotide in chronic pain patients. J. Clin. Pharmacol. 2003, 43, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Byetta™ (Exenatide) Injection. Professional Prescribing Information; Amylin Pharmaceuticals, Inc.: San Diego, CA, USA, 2005. [Google Scholar]
- Cirincione, B.; Mager, D.E. Population pharmacokinetics of exenatide. Br. J. Clin. Pharmacol. 2017, 83, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Giorda, C.B.; Nada, E.; Tartaglino, B. Pharmacokinetics, safety, and efficacy of DPP-4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. A systematic review of the literature. Endocrine 2014, 46, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Pharmacokinetics in patients with chronic liver disease and hepatic safety of incretin-based therapies for the management of type 2 diabetes mellitus. Clin. Pharmacokinet. 2014, 53, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Linnebjerg, H.; Kothare, P.A.; Seger, M.; Wolka, A.M.; Mitchell, M.I. Exenatide-pharmacokinetics, pharmacodynamics, safety and tolerability in patients ≥75 years of age with Type 2 diabetes. Int. J. Clin. Pharmacol. Ther. 2011, 49, 99–108. [Google Scholar] [CrossRef]
- Fineman, M.; Flanagan, S.; Taylor, K.; Aisporna, M.; Shen, L.Z.; Mace, K.F.; Walsh, B.; Diamant, M.; Cirincione, B.; Kothare, P.; et al. Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing. Clin. Pharmacokinet. 2011, 50, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Knop, F.K.; Brønden, A.; Vilsbøll, T. Exenatide: Pharmacokinetics, clinical use, and future directions. Expert Opin. Pharmacother. 2017, 18, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Cirincione, B.; Edwards, J.; Mager, D.E. Population Pharmacokinetics of an Extended-Release Formulation of Exenatide Following Single- and Multiple-Dose Administration. AAPS J. 2017, 19, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Adlyxin (Lixisenatide) Injection, for Subcutaneous Use. Highlights of Prescribing Information; Sanofi-Aventis US LLC: Bridgewaterm, NJ, USA, 2016. [Google Scholar]
- Frank, T. Population Pharmacokinetics of Lixisenatide, a Once-Daily Human Glucagon-Like Peptide-1 Receptor Agonist, in Healthy Subjects and in Patients with Type 2 Diabetes. J. Pharm. Drug Deliv. Res. 2013, 2. [Google Scholar] [CrossRef]
- Raccah, D.; Miossec, P.; Esposito, V.; Niemoeller, E.; Cho, M.; Gerich, J. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: An analysis from the GetGoal phase III programme. Diabetes Metab. Res. Rev. 2015, 31, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Linzess (Linaclotide) Capsules, for Oral Use. Highlights of Prescribing Information; Forest Laboratories, Inc.: St. Louis, MO, USA, 2012. [Google Scholar]
- Lee, N.; Wald, A. The pharmacokinetics, pharmacodynamics, clinical efficacy, safety and tolerability of linaclotide. Expert Opin. Drug Metab. Toxicol. 2011, 7, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Love, B.L.; Johnson, A.; Smith, L.S. Linaclotide: A novel agent for chronic constipation and irritable bowel syndrome. Am. J. Health Syst. Pharm. 2014, 71, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Linaclotide: A review of its use in the treatment of irritable bowel syndrome with constipation. Drugs 2014, 74, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Busby, R.W.; Kessler, M.M.; Bartolini, W.P.; Bryant, A.P.; Hannig, G.; Higgins, C.S.; Solinga, R.M.; Tobin, J.V.; Wakefield, J.D.; Kurtz, C.B.; et al. Pharmacologic properties, metabolism, and disposition of linaclotide, a novel therapeutic peptide approved for the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation. J. Pharmacol. Exp. Ther. 2013, 344, 196–206. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Constella (linaclotide): Summary of Product Characteristics. 2013. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002490/WC500135622.pdf (accessed on 19 October 2018).
- Trulance (plecanatide) Tablets, for Oral Use. Highlights of Prescribing Information; Synergy Pharmaceuticals Inc.: New York, NY, USA, 2017. [Google Scholar]
- Kamuda, J.A.; Mazzola, N. Plecanatide (Trulance) for Chronic Idiopathic Constipation and Irritable Bowel Syndrome with Constipation. Pharmacol. Ther. 2018, 43, 207–232. [Google Scholar]
- Shailubhai, K.; Comiskey, S.; Foss, J.A.; Feng, R.; Barrow, L.; Comer, G.M.; Jacob, G.S. Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig. Dis. Sci. 2013, 58, 2580–2586. [Google Scholar] [CrossRef] [PubMed]
| Drug | Derives From | Mechanism of Action | Major Indication | Innovator Product/s | Major Doses and Formulation/s | Administration Route | Innovator Company | Approved for Clinical Use in |
|---|---|---|---|---|---|---|---|---|
| Captopril | Bradykinin-potentiating factor from the venom of a lancehead viper (Bothrops jararaca) | angiotensin-converting enzyme (ACE) inhibitor | hypertension | Capoten | 6.25 mg, 12.5 mg, 25 mg, 50 mg, and 100 mg tablets | PO | Bristol Myers Squibb | 1981 |
| Cyclosporine | Tolypocladium inflatum fungus | calcineurin inhibitor | immunosuppression | Sandimmune, Neoral | 10 mg, 25 mg, and 100 mg solution or capsules | PO | Novartis | 1983 |
| Eptifibatide | Pigmy rattlesnake (Sistrurus miliarius) | reversible antagonist of the platelet glycoprotein (GP) IIb/IIIa receptor | platelet aggregation inhibition | Integrilin | 0.75 mg and 2 mg solution | IV | Merck Ltd. | 1999 |
| Lepirudin | European medicinal leech (Hirudo medicinalis) | thrombin inhibitor | prevention of thrombosis | Refludan | 20 mg and 50 mg solution | IV | Bayer Healthcare Pharmaceuticals | 1997 (manufacture terminated in 2012) |
| Bivalirudin | thrombin inhibitor | prevention of thrombosis | Angiomax | 250 mg solution | IV | The Medicines Company | 2000 | |
| Ziconotide | Magical cone marine snail (Conus magus) | selective N-type voltage-gated calcium channel blocker | chronic pain | Prialt | 25 ug and 100 ug solution | Intrathecal | Elan Pharmaceuticals | 2004 |
| Exenatide | Gila monster lizard (Heloderma suspectum) | glucagon-like peptide-1 (GLP-1) receptor agonist | type 2 diabetes | Bydureon, Byetta | 5 mg, 10 mg, 250 ug, and 2 mg solution or extended release suspension | SC | Astra Zeneca | 2011 |
| Lixisenatide | glucagon-like peptide-1 (GLP-1) receptor agonist | type 2 diabetes | Adlyxin, Lyxumia | 10 ug, 20 ug, 50 ug, and 100 ug solution | SC | Sanofi-Aventis | 2016 | |
| Linaclotide | Heat-stable enterotoxin from the pathogenic E. coli | guanylate cyclase-C agonist | constipation | Linzess, Constella | 72.5 ug, 145 ug, and 290-ug capsules | PO | Allergan Pharmaceuticals | 2012 |
| Plecanatide | guanylate cyclase-C agonist | constipation | Trulance | 3 mg immediate release tablets | PO | Synergy Pharmaceuticals Inc. | 2017 |
| Drug | MW | Number of Amino Acids and Structural Features | Physiological Charge | logP | Water Solubility mg/mL |
|---|---|---|---|---|---|
| Captopril | 217 | 1, with coordinating sulfhydryl-containing moiety | −1 | 0.34 | 4.52 |
| Cyclosporine | 1203 | 11, cyclic | - | 3.64 | 0.04 |
| Eptifibatide | 832 | 7, cyclic | - | - | 1 |
| Lepirudin | 6963 | 65, with 3 disulfide bridges | - | - | freely soluble |
| Bivalirudin | 2180 | 20 | −4 | −0.76 | 0.0464 |
| Ziconotide | 2639 | 25, with 3 disulfide bridges | - | - | freely soluble |
| Exenatide | 4187 | 39 | - | - | 3 |
| Lixisenatide | 4910 | 44 | −6 | 4.15 | 6 |
| Linaclotide | 1527 | 14, with 3 disulfide bridges | −1 | −1.5 | 0.701 |
| Plecanatide | 1682 | 16, with 2 disulfide bridges | −8 | 0.64 | 0.165 |
| Drug | Absolute Bioavailability, F | Volume of Distribution, V or Apparent V (V/F) L | fu % | Clearance, CL or Apparent CL (CL/F) L/h | t1/2 h | Tmax h |
|---|---|---|---|---|---|---|
| Captopril | 60–75% (PO) | 56 | 65–70 | 49 | 2 | 0.75–1 |
| Cyclosporine | 10–89% (variable, PO) | 210–350 | 10 | 21–29 | 5–27 | 1.5–2.0 |
| Eptifibatide | 100% (IV) | 13–18 (coronary artery disease)15.4–19 (healthy) | 75 | 3.85 | 2.5 | - |
| Lepirudin | 100% (IV) | 12.2 | - | 9.8 | 1.3 | - |
| Bivalirudin | 100% (IV) | 14 | - | 14.3 | 0.42 | - |
| Ziconotide | 50% (intrathecal) | 0.155 | - | - | 2.9–6.5 | - |
| Exenatide | 100% (SC) | 28.3 | - | 9.1 | 2.4 | 2.1 |
| Lixisenatide | -(SC) | 100 | 45 | 35 | 3 | 1–3.5 |
| Linaclotide | ~0% (PO, local activity in the GI, is not absorbed systemically) | - | - | - | - | - |
| Plecanatide | ~0% (PO local activity in the GI, is not absorbed systemically) | - | - | - | - | - |
| Drug | Variability Factor | Dose Adjustment |
|---|---|---|
| Captopril | dimerization and interaction with endogenous thiol-containing compounds in the plasma | - |
| active tubular secretion in the kidneys | dose reduction in renal insufficiency | |
| Cyclosporine | body weight | therapeutic monitoring of trough blood concentrations dosage adjustment taking into account the variability factors |
| co-administration of inhibitors of CYP3A | ||
| hematocrit | ||
| and additional factors | ||
| Eptifibatide | renal elimination of the drug | maintain the IV bolus component and reduce the IV infusion component of the dosage regimen in patients with renal insufficiency |
| Lepirudin | renal elimination of the drug | dose selection based on patients’ weight dose reduction in renal insufficiency monitoring of anticoagulant effects (aPTT test) |
| gender, age, and disease state affect the drug distribution and elimination | ||
| Bivalirudin | renal elimination of the drug | dose selection based on patients’ weight dose reduction in renal insufficiency monitoring of anticoagulant effects (aPTT test) |
| Ziconotide | not reported | - |
| Exenatide | body weight | - |
| renal elimination of the drug | dose reduction in renal insufficiency | |
| Lixisenatide | body weight | - |
| renal elimination of the drug | no dose reduction, but close monitoring of drug safety, in mild or moderate renal impairment; use of the drug in patients with end stage renal disease is not recommended | |
| Linaclotide | not reported | - |
| Plecanatide | not reported | - |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepensky, D. Pharmacokinetics of Toxin-Derived Peptide Drugs. Toxins 2018, 10, 483. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10110483
Stepensky D. Pharmacokinetics of Toxin-Derived Peptide Drugs. Toxins. 2018; 10(11):483. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10110483
Chicago/Turabian StyleStepensky, David. 2018. "Pharmacokinetics of Toxin-Derived Peptide Drugs" Toxins 10, no. 11: 483. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10110483





