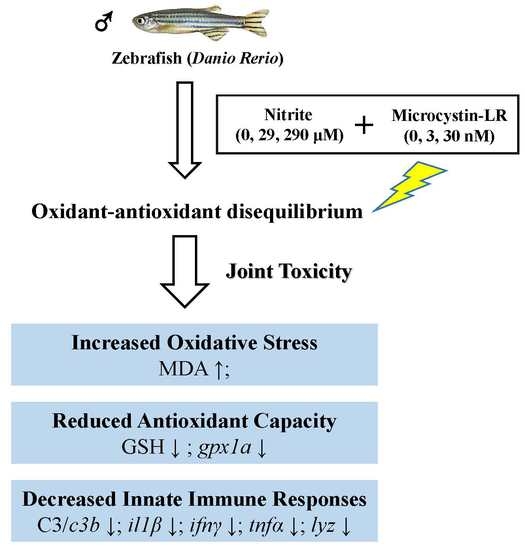
Nitrite Enhances MC-LR-Induced Changes on Splenic Oxidation Resistance and Innate Immunity in Male Zebrafish
Abstract
:1. Introduction
2. Results
2.1. Nitrite and MC-LR and Concentrations in Water Samples
2.2. Spleen Index
2.3. The Levels of LPO, T-AOC and GSH in the Spleen
2.4. Serum Complement C3 Content
2.5. mRNA Expression Profiles of Antioxidant and Innate Immune-Related Genes in the Spleen
2.6. Splenic Pathological Observation
2.7. Correlation Analysis
3. Discussion
3.1. Effects of Nitrite and MC-LR on Splenic Oxidant-Antioxidant Status
3.2. Effects of Nitrite and MC-LR on Innate Immune Responses
3.3. Effects of Nitrite and MC-LR on Splenic Pathological Changes
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Zebrafish Maintenance, Exposure and Sampling
5.3. Biochemical Parameter Analysis
5.4. Gene Transcription Analysis
5.5. Pathological Studies
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wert, E.C.; Rosario-Ortiz, F.L. Intracellular organic matter from cyanobacteria as a precursor for carbonaceous and nitrogenous disinfection byproducts. Environ. Sci. Technol. 2013, 47, 6332–6340. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Shao, Y.; Gao, N.; Deng, Y.; Li, L.; Deng, J.; Tan, C. Characterization of algal organic matters of Microcystis aeruginosa: Biodegradability, DBP formation and membrane fouling potential. Water Res. 2014, 52, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yan, W.; Wu, Q.; Lu, J.; Liu, C.; Hung, T.C.; Li, G. Adverse reproductive performance in zebrafish with increased bioconcentration of microcystin-LR in the presence of titanium dioxide nanoparticles. Environ. Sci. Nano 2018, 5, 1208–1217. [Google Scholar] [CrossRef]
- Song, W.; De La Cruz, A.A.; Rein, K.; O’Shea, K.E. Ultrasonically induced degradation of microcystin-LR and-RR: Identification of products, effect of pH, formation and destruction of peroxides. Environ. Sci. Technol. 2006, 40, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Triantis, T.M.; Fotiou, T.; Kaloudis, T.; Kontos, A.G.; Falaras, P.; Dionysiou, D.D.; Pelaez, M.; Hiskia, A. Photocatalytic degradation and mineralization of microcystin-LR under UV-A, solar and visible light using nanostructured nitrogen doped TiO2. J. Hazard. Mater. 2012, 211, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zastepa, A.; Pick, F.R.; Blais, J.M.; Saleem, A. Analysis of intracellular and extracellular microcystin variants in sediments and pore waters by accelerated solvent extraction and high performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 872, 26–34. [Google Scholar] [CrossRef]
- Lahti, K.; Rapala, J.; Färdig, M.; Niemelä, M.; Sivonen, K. Persistence of cyanobacterial hepatotoxin, microcystin-LR in particulate material and dissolved in lake water. Water Res. 1997, 31, 1005–1012. [Google Scholar] [CrossRef]
- Teng, W.; Wu, Z.; Feng, D.; Fan, J.; Wang, J.; Wei, H.; Song, M.; Zhao, D. Rapid and efficient removal of microcystins by ordered mesoporous silica. Environ. Sci. Technol. 2013, 47, 8633–8641. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, Y.; Xie, P.; Chen, J.; Ma, Z.; Tao, M.; Qi, M.; Wu, L.; Guo, L. Factors affecting temporal and spatial variations of microcystins in Gonghu Bay of Lake Taihu, with potential risk of microcystin contamination to human health. Sci. World J. 2010, 10, 1795–1809. [Google Scholar] [CrossRef]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Xiang, F.; Minter, E.J.; Lü, K.; Chen, Y.; Montagnes, D.J. The interactive effects of microcystin and nitrite on life-history parameters of the cladoceran Daphnia obtusa. J. Hazard. Mater. 2011, 190, 113–118. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (US EPA). Role of Bacteria in the Nitrogen Cycle in Lakes; Water Pollution Control Series (16010, EHR 03/72); Water Pollution Control: Washington, DC, USA, 1972; p. 23.
- Woo, N.Y.S.; Chiu, S.F. Effects of nitrite exposure on growth and survival of sea bass, Lates calcarifer, fingerlings in various salinities. J. Appl. Aquac. 1995, 4, 45–54. [Google Scholar] [CrossRef]
- Jensen, F.B. Nitrite disrupts multiple physiological functions in aquatic animals. Comp. Biochem. Physiol. A 2003, 135, 9–24. [Google Scholar] [CrossRef]
- Madison, B.N.; Wang, Y.S. Haematological responses of acute nitrite exposure in walleye (Sander vitreus). Aquat. Toxicol. 2006, 79, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Deane, E.E.; Woo, N.Y. Impact of nitrite exposure on endocrine, osmoregulatory and cytoprotective functions in the marine teleost Sparus sarba. Aquat. Toxicol. 2007, 82, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Lee, J.; Hur, J.W.; Song, Y.C.; Na, H.C.; Noh, C.H. Acute toxicity and sublethal effects of nitrite on selected hematological parameters and tissues in dark-banded rockfish, Sebastes inermis. J. World Aquac. Soc. 2007, 38, 188–199. [Google Scholar] [CrossRef]
- Long, M.; Lin, W.; Hou, J.; Guo, H.H.; Li, L.; Li, D.P.; Tang, R.; Yang, F. Dietary supplementation with selenium yeast and tea polyphenols improve growth performance and nitrite tolerance of Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2017, 68, 74–83. [Google Scholar] [CrossRef]
- Amado, L.L.; Monserrat, J.M. Oxidative stress generation by microcystins in aquatic animals: Why and how. Environ. Int. 2010, 36, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm Filho, D. Fish antioxidant defenses—A comparative approach. Braz. J. Med. Biol. Res. 1996, 29, 1735–1742. [Google Scholar] [PubMed]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, P.; Chen, J. Biochemical and ultrastructural changes of the liver and kidney of the phytoplanktivorous silver carp feeding naturally on toxic Microcystis blooms in Taihu Lake, China. Toxicon 2007, 49, 1042–1053. [Google Scholar] [CrossRef]
- Atencio, L.; Moreno, I.; Jos, A.; Pichardo, S.; Moyano, R.; Blanco, A.; Cameán, A.M. Dose-dependent antioxidant responses and pathological changes in tenca (Tinca tinca) after acute oral exposure to Microcystis under laboratory conditions. Toxicon 2008, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, L.; Xue, T.; Long, M.; Su, Y.; Wu, N. Damage and recovery of the ovary in female zebrafish ip-injected with MC-LR. Aquat. Toxicol. 2014, 155, 110–118. [Google Scholar] [CrossRef]
- Sun, S.; Ge, X.; Xuan, F.; Zhu, J.; Yu, N. Nitrite-induced hepatotoxicity in bluntsnout bream (Megalobrama amblycephala): The mechanistic insight from transcriptome to physiology analysis. Environ. Toxicol. Pharmacol. 2014, 37, 55–65. [Google Scholar] [CrossRef]
- Meydani, S.N.; Wu, D.; Santos, M.S.; Hayek, M.G. Antioxidants and immune response in aged persons: Overview of present evidence. Am. J. Clin. Nutr. 1995, 62, 1462S–1476S. [Google Scholar] [CrossRef]
- Baier-Bitterlich, G.; Fuchs, D.; Wachter, H. Chronic immune stimulation, oxidative stress, and apoptosis in HIV infection. Biochem. Pharmacol. 1997, 53, 755–763. [Google Scholar] [CrossRef]
- Zapata, A.; Diez, B.; Cejalvo, T.; Gutierrez-de Frias, C.; Cortes, A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006, 20, 126–136. [Google Scholar] [CrossRef]
- Djediat, C.; Malécot, M.; de Luze, A.; Bernard, C.; Puiseux-Dao, S.; Edery, M. Localization of microcystin-LR in medaka fish tissues after cyanotoxin gavage. Toxicon 2010, 55, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Liang, H.; Zhang, X. Effect of cyanobacteria on immune function of crucian carp (Carassius auratus) via chronic exposure in diet. Chemosphere 2013, 90, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.; Wei, X.; Xu, M.; Huang, C.; Wang, W.; Wang, H. Characterization of a novel CC chemokine CCL4 in immune response induced by nitrite and its expression differences among three populations of Megalobrama amblycephala. Fish Shellfish Immunol. 2014, 38, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Liu, B.L.; Han, C.; Huang, B.; Lei, J.L. The physiological performance and immune response of juvenile turbot (Scophthalmus maximus) to nitrite exposure. Comp. Biochem. Physiol. C 2016, 181, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Chand, R.K.; Sahoo, P.K. Effect of nitrite on the immune response of freshwater prawn Macrobrachium malcolmsonii and its susceptibility to Aeromonas hydrophila. Aquaculture 2006, 258, 150–156. [Google Scholar] [CrossRef]
- Yang, Z.; Lü, K.; Chen, Y.; Montagnes, D.J. The interactive effects of ammonia and microcystin on life-history traits of the cladoceran Daphnia magna: Synergistic or antagonistic? PLOS ONE 2012, 7, e32285. [Google Scholar] [CrossRef] [PubMed]
- Christin, M.S.; Gendron, A.D.; Brousseau, P.; Menard, L.; Marcogliese, D.J.; Cyr, D.; Ruby, S.; Fournier, M. Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environ. Toxicol. Chem. 2003, 22, 1127–1133. [Google Scholar] [CrossRef]
- Jiang, J.; Su, M.; Chen, Y.; Gao, N.; Jiao, C.; Sun, Z.; Li, F.; Wang, C. Correlation of drought resistance in grass pea (Lathyrus sativus) with reactive oxygen species scavenging and osmotic adjustment. Biologia 2013, 68, 231–240. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, J.; Shan, Z.; Bu, Y.; Deng, Z.; Cheng, Y. Oxidative stress and histopathological alterations in liver of Cyprinus carpio L. induced by intraperitoneal injection of microcystin-LR. Ecotoxicology 2015, 24, 511–519. [Google Scholar] [CrossRef]
- Amado, L.L.; Garcia, M.L.; Ramos, P.B.; Freitas, R.F.; Zafalon, B.; Ferreira, J.L.R.; Yunes, J.S.; Monserrat, J.M. A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: Application to evaluate microcystins toxicity. Sci. Total Environ. 2009, 407, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; He, D.; Yan, F.; Liang, F. Effects of NaNO2 on malondialdehyde content and total antioxidative capacity in the liver of Carassius auratus. J. Agro-Environ. Sci. 2005, 24, 21–24. (In Chinese) [Google Scholar]
- Wang, W.N.; Wang, A.L.; Zhang, Y.J.; Li, Z.H.; Wang, J.X.; Sun, R.Y. Effects of nitrite on lethal and immune response of Macrobrachium nipponense. Aquaculture 2004, 232, 679–686. [Google Scholar] [CrossRef]
- Hou, J.; Li, L.; Xue, T.; Long, M.; Su, Y.; Wu, N. Hepatic positive and negative antioxidant responses in zebrafish after intraperitoneal administration of toxic microcystin-LR. Chemosphere 2015, 120, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, C.; Yun, W.; Yang, Z.; Chen, Y.; Zuo, Z. Antioxidant responses to benzo[a]pyrene, tributyltin and their mixture in the spleen of Sebasticus marmoratus. J. Environ. Sci. 2007, 19, 1129–1135. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Song, L.; Liu, J. Responses of antioxidant systems in the hepatocytes of common carp (Cyprinus carpio L.) to the toxicity of microcystin-LR. Toxicon 2003, 42, 85–89. [Google Scholar] [CrossRef]
- Jiang, J.; Gu, X.; Song, R.; Zhang, Q.; Geng, J.; Wang, X.; Yang, L. Time-dependent oxidative stress and histopathological changes in Cyprinus carpio L. exposed to microcystin-LR. Ecotoxicology 2011, 20, 1000–1009. [Google Scholar] [CrossRef]
- Jia, R.; Han, C.; Lei, J.L.; Liu, B.L.; Huang, B.; Huo, H.H.; Yin, S.T. Effects of nitrite exposure on haematological parameters, oxidative stress and apoptosis in juvenile turbot (Scophthalmus maximus). Aquat. Toxicol. 2015, 169, 1–9. [Google Scholar] [CrossRef]
- Demers, N.E.; Bayne, C.J. The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 1997, 21, 363–373. [Google Scholar] [CrossRef]
- Boshra, H.; Li, J.; Sunyer, J.O. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 2006, 20, 239–262. [Google Scholar] [CrossRef]
- Chipman, D.M.; Sharon, N. Mechanism of lysozyme action. Science 1969, 165, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Jollès, P.; Jollès, J. What’s new in lysozyme research? Mol. Cell. Biochem. 1984, 63, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.C.H.; Lambris, J.D. The complement system in teleosts. Fish Shellfish Immunol. 2002, 12, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, X.; Wang, J.; Li, C. Effect of pure microcystin-LR on activity and transcript level of immune-related enzymes in the white shrimp (Litopenaeus vannamei). Ecotoxicology 2017, 26, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Ciji, A.; Sahu, N.P.; Pal, A.K.; Akhtar, M.S. Dietary L-tryptophan modulates growth and immune-metabolic status of Labeo rohita juveniles exposed to nitrite. Aquac. Res. 2015, 46, 2013–2024. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T.; Hong, S.; Peddie, S.; Crampe, M.; Laing, K.J.; Cunningham, C.; Zou, J. Cytokines and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 713–723. [Google Scholar] [CrossRef]
- Arango, D.G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Rymuszka, A.; Adaszek, Ł. Cytotoxic effects and changes in cytokine gene expression induced by microcystin-containing extract in fish immune cells—An in vitro and in vivo study. Fish Shellfish Immunol. 2013, 34, 1524–1532. [Google Scholar] [CrossRef]
- Wei, L.; Sun, B.; Chang, M.; Liu, Y.; Nie, P. Effects of cyanobacterial toxin microcystin-LR on the transcription levels of immune-related genes in grass carp Ctenopharyngodon idella. Environ. Biol. Fish. 2009, 85, 231. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, R.; Liu, W.; Fu, Z. Effect of endocrine disrupting chemicals on the transcription of genes related to the innate immune system in the early developmental stage of zebrafish (Danio rerio). Fish Shellfish Immunol. 2010, 28, 854–861. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.A. Free radicals, antioxidants, and the immune system. Ann. Clin. Lab. Sci. 2000, 30, 145–158. [Google Scholar] [PubMed]
- De la Fuente, M. Effects of antioxidants on immune system ageing. Eur. J. Clin. Nutr. 2002, 56, S5. [Google Scholar] [CrossRef] [PubMed]
- Eze, M.O. Membrane fluidity, reactive oxygen species, and cell-mediated immunity: Implications in nutrition and disease. Med. Hypotheses 1992, 37, 220–224. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.C.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Inmunología 2003, 22, 277–286. [Google Scholar]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Rijkers, G.T.; Frederix-Wolters, E.M.H.; Van Muiswinkel, W.B. The haemolytic plaque assay in carp (Cyprinus carpio). J. Immunol. Methods 1980, 33, 79–86. [Google Scholar] [CrossRef]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1. [Google Scholar] [CrossRef]
- Palíková, M.; Kovářů, F.; Navratil, S.; Kubala, L.; Pešák, S.; Vajcová, V. The effect of pure microcystin LR and biomass of blue-green algae on selected immunological indices of carp (Cyprinus carpio L.) and silver carp (Hypophthalmichthys molitrix Val.). Acta Vet. Brno 1998, 67, 265–272. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondria: Dynamic organelles in disease, aging, and development. Cell 2006, 125, 1241–1252. [Google Scholar] [CrossRef]
- Grosell, M.; Jensen, F.B. Uptake and effects of nitrite in the marine teleost fish Platichthys flesus. Aquat. Toxicol. 2000, 50, 97–107. [Google Scholar] [CrossRef]
- Alonso, A.; Camargo, J.A. Toxicity of nitrite to three species of freshwater invertebrates. Environ. Toxicol. 2006, 21, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Voslářová, E.; Pištěková, V.; Svobodova, Z.; Bedáňová, I. Nitrite toxicity to Danio rerio: Effects of subchronic exposure on fish growth. Acta Vet. Brno 2008, 77, 455–460. [Google Scholar] [CrossRef]
- Hou, J.; Li, L.; Wu, N.; Su, Y.; Lin, W.; Li, G.; Gu, Z. Reproduction impairment and endocrine disruption in female zebrafish after long-term exposure to MC-LR: A life cycle assessment. Environ. Pollut. 2016, 208, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, P.K.; Rathore, D.P.S. Spectrophotometric determination of nitrite in water. Analyst 1988, 113, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Qin, J.; Qin, C.; Li, E.; Jiang, H. Effects of dietary soybean oil inclusion to replace fish oil on growth, muscle fatty acid composition, and immune responses of juvenile darkbarbel catfish, Pelteobagrus vachelli. Afr. J. Agric. Res. 2013, 8, 1492–1499. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, L.; Hou, J.; Wu, N.; Lin, W.; Li, G. Life-cycle exposure to microcystin-LR interferes with the reproductive endocrine system of male zebrafish. Aquat. Toxicol. 2016, 175, 205–212. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Source of Variation | df | F | p |
|---|---|---|---|---|
| Spleen index | Nitrite | 2 | 4.017 | 0.025 |
| MC-LR | 2 | 5.145 | 0.010 | |
| Nitrite × MC-LR | 4 | 0.253 | 0.907 | |
| MDA | Nitrite | 2 | 12.707 | <0.001 |
| MC-LR | 2 | 8.872 | <0.001 | |
| Nitrite × MC-LR | 4 | 1.539 | 0.042 | |
| T-AOC | Nitrite | 2 | 6.872 | 0.002 |
| MC-LR | 2 | 9.512 | <0.001 | |
| Nitrite × MC-LR | 4 | 0.282 | 0.888 | |
| GSH | Nitrite | 2 | 2.679 | 0.040 |
| MC-LR | 2 | 3.792 | 0.030 | |
| Nitrite × MC-LR | 4 | 3.070 | 0.026 | |
| C3 | Nitrite | 2 | 90.553 | <0.001 |
| MC-LR | 2 | 15.423 | <0.001 | |
| Nitrite × MC-LR | 4 | 4.814 | 0.005 |
| Genes | Source of Variation | df | F | p |
|---|---|---|---|---|
| cat1 | Nitrite | 2 | 2.249 | 0.117 |
| MC-LR | 2 | 7.856 | 0.001 | |
| Nitrite × MC-LR | 4 | 1.313 | 0.280 | |
| sod1 | Nitrite | 2 | 1.035 | 0.281 |
| MC-LR | 2 | 12.663 | <0.001 | |
| Nitrite × MC-LR | 4 | 1.999 | 0.111 | |
| gpx1a | Nitrite | 2 | 11.258 | <0.001 |
| MC-LR | 2 | 24.901 | <0.001 | |
| Nitrite × MC-LR | 4 | 3.886 | 0.009 | |
| il1β | Nitrite | 2 | 2.331 | 0.109 |
| MC-LR | 2 | 24.154 | <0.001 | |
| Nitrite × MC-LR | 4 | 2.744 | 0.040 | |
| ifnγ | Nitrite | 2 | 19.295 | <0.001 |
| MC-LR | 2 | 39.856 | <0.001 | |
| Nitrite × MC-LR | 4 | 11.660 | <0.001 | |
| tnfα | Nitrite | 2 | 15.468 | <0.001 |
| MC-LR | 2 | 6.907 | 0.002 | |
| Nitrite × MC-LR | 4 | 2.962 | 0.030 | |
| c3b | Nitrite | 2 | 12.151 | <0.001 |
| MC-LR | 2 | 58.140 | <0.001 | |
| Nitrite × MC-LR | 4 | 2.593 | 0.049 | |
| lyz | Nitrite | 2 | 10.311 | <0.001 |
| MC-LR | 2 | 15.744 | <0.001 | |
| Nitrite × MC-LR | 4 | 5.762 | 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Guo, H.; Wang, L.; Zhang, D.; Wu, X.; Li, L.; Li, D.; Tang, R. Nitrite Enhances MC-LR-Induced Changes on Splenic Oxidation Resistance and Innate Immunity in Male Zebrafish. Toxins 2018, 10, 512. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10120512
Lin W, Guo H, Wang L, Zhang D, Wu X, Li L, Li D, Tang R. Nitrite Enhances MC-LR-Induced Changes on Splenic Oxidation Resistance and Innate Immunity in Male Zebrafish. Toxins. 2018; 10(12):512. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10120512
Chicago/Turabian StyleLin, Wang, Honghui Guo, Lingkai Wang, Dandan Zhang, Xueyang Wu, Li Li, Dapeng Li, and Rong Tang. 2018. "Nitrite Enhances MC-LR-Induced Changes on Splenic Oxidation Resistance and Innate Immunity in Male Zebrafish" Toxins 10, no. 12: 512. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins10120512