Mycotoxins and Related Fungi in Italian Paddy Rice During the Growing Season and Storage
Abstract
:1. Introduction
2. Results and Discussion
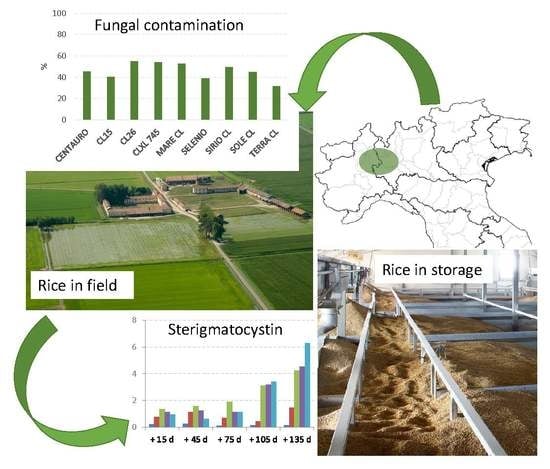
2.1. Mycotoxigenic Fungi Contamination in the Field
2.2. Mycotoxin Occurrence in Field
2.3. Fungal Contamination during Storage
2.4. Mycotoxin Contamination during Storage
3. Conclusions
4. Materials and Methods
4.1. Field Samples
4.2. Storage
- Medium refrigeration (MR) at 11–15 °C in a conventional warehouse (2 rice varieties: Sirio CL and Mare CL)
- Room temperature (RT) in a conventional warehouse (2 rice varieties: Sole CL and Centauro)
4.3. Fungal Identification
4.4. Reagents and Standards
4.5. Analysis for Mycotoxin Determination
4.6. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ente Nazionale Risi. 2018. Available online: www.enterisi.it (accessed on 28 November 2018).
- Reddy, K.; Reddy, C.; Muralidharan, K. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 2009, 26, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, M. Historical review of researches on yellow rice and mycotoxigenic fungi adherent to rice in Japan. Jpn. Soc. Mycotoxicol. (JSM) Mycotoxins 2015, 65, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Maheshwar, P.K.; Janardhana, G.R. Natural occurrence of toxigenic Fusarium proliferatum on paddy (Oryza sativa L.) in Karnataka, India. Trop. Life Sci. Res. 2010, 21, 1–10. [Google Scholar] [PubMed]
- Jettanajit, A.; Nhujak, T. Determination of mycotoxins in brown rice using Quechers sample preparation and UHPLC-MS-MS. J. Chromatogr. Sci. 2016, 54, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z.; Asi, M.R.; Hanif, U.; Zuber, M.; Jinap, S. The presence of aflatoxins and ochratoxin A in rice and rice products and evaluation of dietary intake. Food Chem. 2016, 210, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Katsurayama, A.M.; Martins, L.M.; Iamanaka, B.T.; Fungaro, M.H.P.; Silva, J.J.; Taniwaki, M.H. Occurrence of Aspergillus section Flavi and aflatoxin in Brasil: From field to market. Int. J. Food Microbiol. 2018, 266, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Su, P.; Shan, H. Mycotoxin contamination of rice in China. J. Food Sci. 2017, 82, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Rofiat, A.S.; Fanelli, F.; Atanda, O.; Sulyok, M.; Gozzi, G.; Bavaro, S.; Krska, R.; Logrieco, A.F.; Ezekiel, C.N. Fungal and bacterial metabolites associated with natural contamination of locally processed rice (Oryza sativa L.) in Nigeria. Food Addit. Contam. A 2015, 32, 950–959. [Google Scholar] [CrossRef] [PubMed]
- EU. Commission Regulation (EU) No. 165/2010 of 26 February 2010 amending Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union 2010, L50, 8–12. [Google Scholar]
- EU. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Mol, H.G.; Mac Donald, S.; Anagnostopoulos, C.; Spanjer, M.; Bertuzzi, T.; Pietri, A. European survey on sterigmatocystin in cereals, cereals-based products, beer and nuts. World Mycotoxin J. 2016, 9, 633–642. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Romani, M.; Rastelli, S.; Mulazzi, A.; Pietri, A. Sterigmatocystin occurrence in paddy and processed rice produced in Italy in the years 2014–2015 and distribution in milled rice fractions. Toxins 2017, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Abramson, D.; Hulasareb, R.; Whitea, N.D.G.; Jayasb, D.S.; Marquardt, R.R. Mycotoxin formation in hulless barley during granary storage at 15 and 19% moisture content. J. Stored Prod. Res. 1999, 35, 297–305. [Google Scholar] [CrossRef]
- Atalla, M.M.; Hassanein, N.M.; El-Beih, A.A.; Youssef, Y.A. Mycotoxin production in wheat grains by different Aspergilli in relation to different relative humidities and storage periods. Nahrung 2003, 47, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Leggieri, M.C.; Decontardi, S.; Bertuzzi, T.; Pietri, A.; Battilani, P. Modeling growth and toxin production of toxigenic fungi signaled in cheese under different temperature and water activity regimes. Toxins 2017, 9, 1–4. [Google Scholar]
- Gonçalves, A.; Gkrillas, A.; Dorne, J.L.; Dall’Asta, C.; Palumbo, R.; Lima, N.; Battilani, P.; Venâncio, A.; Giorni, P. Pre- and Postharvest Strategies to Minimize Mycotoxin Contamination in the Rice Food Chain. Compr. Rev. Food Sci. Food Saf. 2019, 18, 441–454. [Google Scholar] [CrossRef]
- Savi, G.D.; Piacentini, K.C.; Rocha, L.O.; Carnielli-Queiroz, L.; Furtado, B.G.; Scussel, R.; Zanoni, E.T.; Machado-de-Avila, R.A.; Correa, B.; Angioletto, E. Incidence of toxigenic fungi and zearalenone in rice grains from Brazil. Int. J. Food Microbiol. 2018, 270, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.C.; Yoshizawa, T. Mold counts and Aspergillus section Flavi populations in rice and its by-products from the Philippines. J. Food Prot. 2005, 68, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Giorni, P.; Dall’Asta, C.; Gregori, R.; Cirlini, M.; Galaverna, G.; Battilani, P. Starch and thermal treatment, important factors in changing detectable fumonisins in maize post-harvest. J. Cereal Sci. 2015, 61, 78–85. [Google Scholar] [CrossRef]
- Singh, M.K.; Sinha, K.K. Levels of aflatoxin B1 production in seeds of some selected varieties of paddy and their relation with total starch, amylose and amylopectin contents. Int. J. Life Sci. Biotechnol. Pharma Res. 2013, 2, 222–226. [Google Scholar]
- FAO. JEFCA Monographs; World Health Organizaton (WHO): Geneva, Switzerland, 2018. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11, 3254. [Google Scholar] [CrossRef] [Green Version]
- Díaz Nieto, C.H.; Granero, A.M.; Zon, M.A.; Fernández, H. Sterigmatocystin: A mycotoxin to be seriously considered. Food Chem. Toxicol. 2018, 118, 460–470. [Google Scholar]
- Almeida, M.I.; Almeida, N.G.; Carvalho, K.L.; Gonçalves, G.A.A.; Silva, C.N.; Santos, E.A.; Garcia, J.C.; Vargas, E.A. Co-occurrence of aflatoxins B1, B2, G1 and G2, ochratoxin A, zearalenone, deoxynivalenol, and citreoviridin in rice in Brazil. Food Addit. Contam. A 2012, 29, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Zhou, J.; Huang, B.F.; Cai, Z.X.; Xu, X.M.; Ren, Y.P. Simultaneous and rapid determination of deoxynivalenol and its acetylate derivatives in wheat flour and rice by ultra high performance liquid chromatography with photo diode array detection. J. Sep. Sci. 2016, 39, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Ok, H.E.; Kim, D.M.; Kim, D.; Chung, S.H.; Chung, M.S.; Park, K.H.; Chun, H.S. Mycobiota and natural occurrence of aflatoxin, deoxynivalenol, nivalenol and zearalenone in rice freshly harvested in South Korea. Food Control. 2014, 37, 284–291. [Google Scholar] [CrossRef]
- Ok, H.E.; Lee, S.Y.; Chun, H.S. Occurrence and simultaneous determination of nivalenol and deoxynivalenol in rice and bran by HPLC-UV detection and immunoaffinity cleanup. Food Control 2018, 87, 53–59. [Google Scholar] [CrossRef]
- Ali, N. Co-occurrence of citrinin and ochratoxin A in rice in Asia and its implications for human health. J. Sci. Food Agric. 2017, 98, 2055–2059. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Liu, R.; Ruan, C.; Zhang, H.; Liu, C. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 2015, 50, 401–404. [Google Scholar] [CrossRef]
- Bansal, J.; Pantazopoulos, P.; Tam, J.; Cavlovic, P.; Kwong, K.; Turcotte, A.M.; Lau, B.P.Y.; Scott, P.M. Surveys of rice sold in Canada for aflatoxins, ochratoxin A and fumonisins. Food Addit. Contam. 2011, 28, 767–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. Eur. J. Plant Pathol. 2003, 109, 723–730. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-harvestcontrol strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Chulze, S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam. A 2010, 27, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Raper, K.B.; Fennell, D.I. The Genus Aspergillus; Robert, E., Ed.; Krieger Publishing Company Inc.: Malabar, FL, USA, 1965. [Google Scholar]
- Bertuzzi, T.; Rastelli, S.; Mulazzi, A.; Pietri, A. Evaluation and improvement of extraction methods for the analysis of aflatoxins B1, B2, G1 and G2 from naturally contaminated maize. Food Anal. Method 2012, 5, 512–519. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Leggieri, M.C.; Battilani, P.; Pietri, A. Co-occurrence of type A and B trichothecenes and zearalenone in wheat grown in northern Italy over the years 2009–2011. Food Addit. Contam. B 2014, 7, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Bertuzzi, T.; Comizzoli, S.; Turconi, G.; Roggi, C.; Pagani, M.; Cravedi, P.; Pietri, A. Preliminary survey on composition and quality of conventional and organic wheat. Ital. J. Food Sci. 2006, 4, 355–366. [Google Scholar]
- Clewer, A.G.; Scarisbrick, D.H. Practical Statistics and Experimental Design for Plant and Crop Science; John Wiley & Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]




| Factor | Total Fungi Incidence (%) | Incidence of Fusarium spp. (%) | Incidence of A. versicolor (%) | Incidence of A. flavus (%) | STC (µg/kg) | AFB1 (µg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling time (A) | ** | ** | ** | ** | ** | ** | ||||||
| Flowering (BBCH 69) | 0.3 | e | 0.0 | d | 0.0 | c | 0.0 | b | 0.0 | d | 0.0 | b |
| 15 days post-flowering (BBCH 77) | 15.6 | d | 3.1 | c | 0.7 | bc | 0.8 | a | 0.4 | c | 0.1 | ab |
| Early dough (BBCH 83) | 26.5 | c | 2.2 | c | 1.6 | ab | 0.1 | b | 1.4 | b | 0.1 | ab |
| Full Ripening (BBCH 89) | 89.0 | a | 18.9 | a | 1.3 | ab | 0.1 | b | 2.0 | a | 0.1 | ab |
| Over ripening (BBCH 92) | 67.6 | b | 10.4 | b | 2.2 | a | 0.0 | b | 1.6 | ab | 0.2 | a |
| Rice variety (B) | ** | ** | ** | N.S. | ** | ** | ||||||
| Centauro | 45.6 | a | 11.7 | ab | 0.6 | bc | 0.2 | 1.0 | bcd | 0.0 | b | |
| Selenio | 38.9 | ab | 7.8 | bcd | 0.3 | c | 0.2 | 1.1 | cd | 0.1 | ab | |
| Sole CL | 45.2 | a | 4.8 | cd | 0.7 | bc | 0.0 | 1.9 | a | 0.1 | ab | |
| Terra CL | 31.5 | b | 5.1 | d | 0.2 | c | 0.2 | 0.3 | e | 0.0 | b | |
| CL26 | 55.1 | a | 4.4 | cd | 4.1 | a | 0.0 | 1.9 | ab | 0.3 | a | |
| CLXL 745 | 54.3 | a | 7.3 | abcd | 1.3 | b | 0.5 | 1.6 | abc | 0.2 | ab | |
| Mare CL | 52.5 | a | 4.4 | cd | 1.1 | bc | 0.5 | 2.4 | a | 0.1 | ab | |
| Sirio CL | 49.5 | a | 12.5 | a | 4.8 | a | 0.3 | 0.7 | de | 0.1 | ab | |
| CL15 | 40.3 | ab | 10.5 | abc | 0.2 | c | 0.2 | 1.0 | bcd | 0.0 | b | |
| Sowing density (C) | N.S. | * | N.S. | N.S. | N.S. | N.S. | ||||||
| 100 kg/ha | 65.8 | 8.2 | ab | 2.4 | 0.3 | 1.7 | 0.1 | |||||
| 150kg/ha | 60.3 | 11.1 | a | 2.0 | 0.1 | 1.8 | 0.1 | |||||
| 200kg/ha | 61.2 | 7.5 | b | 2.3 | 7.5 | 1.9 | 0.1 | |||||
| AxB | ** | ** | ** | ** | ** | N.S. | ||||||
| AxC | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | ||||||
| BxC | N.S. | * | ** | * | N.S. | N.S. | ||||||
| AxBxC | N.S. | N.S. | ** | ** | N.S. | N.S. | ||||||
| Factor | Total Fungi Incidence (%) | Incidence of Fusarium spp. (%) | Incidence of A. versicolor (%) | Incidence of A. flavus (%) | STC (µg/kg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Time (A) | N.S. | N.S. | N.S. | N.S. | ** | |||||
| +15 days | 41.1 | 10.8 | 0.1 | 0.0 | 1.2 | b | ||||
| +45 days | 51.0 | 7.7 | 1.2 | 0.1 | 1.3 | b | ||||
| +75 days | 47.1 | 12.4 | 1.8 | 0.0 | 1.3 | b | ||||
| +105 days | 50.3 | 11.7 | 0.4 | 0.0 | 2.4 | ab | ||||
| +135 days | 48.5 | 3.6 | 0.3 | 0.0 | 3.3 | a | ||||
| Storage (B) | ** | N.S. | N.S. | N.S. | N.S. | |||||
| MR | 40.7 | b | 9.6 | 0.8 | 0.0 | 1.7 | ||||
| RT | 68.8 | a | 9.3 | 0.9 | 0.1 | 2.2 | ||||
| AxB | N.S. | N.S. | N.S. | N.S. | N.S. | |||||
| Rice Varieties | Field A | Grain Type | Field B | Grain Type | Field C | Grain Type |
|---|---|---|---|---|---|---|
| 1 | CLXL 745 | LONG B | SOLE CL | ROUND | CL15 | ROUND |
| 2 | CL26 | LONG B | SELENIO | ROUND | SELENIO | ROUND |
| 3 | SIRIO CL | LONG B | CENTAURO | ROUND | CENTAURO | ROUND |
| 4 | MARE CL | LONG B | TERRA CL | ROUND | TERRA CL | ROUND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuzzi, T.; Romani, M.; Rastelli, S.; Giorni, P. Mycotoxins and Related Fungi in Italian Paddy Rice During the Growing Season and Storage. Toxins 2019, 11, 151. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11030151
Bertuzzi T, Romani M, Rastelli S, Giorni P. Mycotoxins and Related Fungi in Italian Paddy Rice During the Growing Season and Storage. Toxins. 2019; 11(3):151. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11030151
Chicago/Turabian StyleBertuzzi, Terenzio, Marco Romani, Silvia Rastelli, and Paola Giorni. 2019. "Mycotoxins and Related Fungi in Italian Paddy Rice During the Growing Season and Storage" Toxins 11, no. 3: 151. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11030151