Characterization of Two Novel Bacillus thuringiensis Cry8 Toxins Reveal Differential Specificity of Protoxins or Activated Toxins against Chrysomeloidea Coleopteran Superfamily
Abstract
:1. Introduction
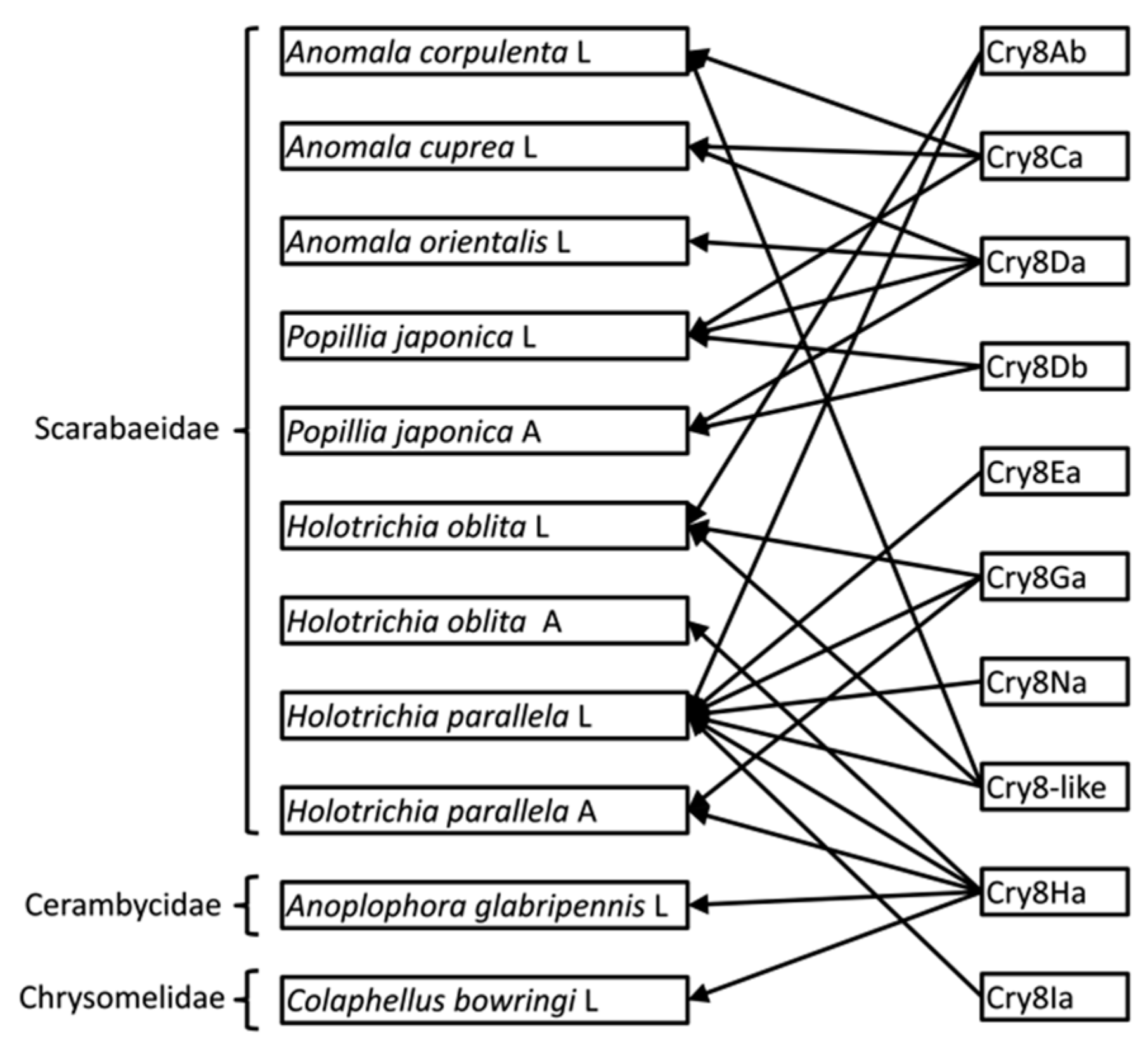
2. Results
2.1. Cloning and Sequence Analysis of cry8Ha1 and cry8Ia1 from BtSU4
2.2. Differential Toxicity of Cry8Ha1 and Cry8Ia1 Protoxins and Activated Toxins Against Different Coleopteran Insects
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacteria Growth Conditions and Plasmids
5.2. Gene Cloning and Sequence Analysis
5.3. Protein Expression, Extraction and Activation
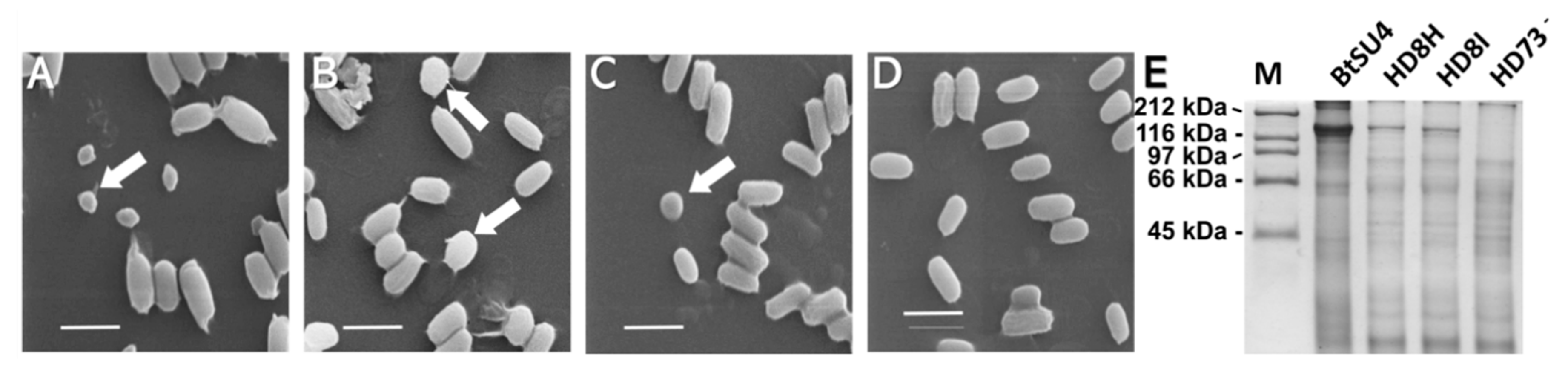
5.4. Scanning Electron Microscopy (SEM) Examination
5.5. Insects, Rearing Conditions and Bioassay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bouchard, P.; Bousquet, Y. Additions and corrections to “Family-group names in Coleoptera (Insecta)”. ZooKeys 2020, 922, 65–139. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, P.; Bousquet, Y.; Davies, A.E.; Alonso-Zarazaga, M.A.; Lawrence, J.F.; Lyal, C.H.; Newton, A.F.; Reid, C.A.; Schmitt, M.; Slipiński, S.A.; et al. Family-group names in Coleoptera (Insecta). ZooKeys 2011, 88, 1–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Chandra, A.; Pandey, K.C. Bacillus thuringiensis (Bt) transgenic crop: An environment friendly insect-pest management strategy. J. Environ. Biol. 2008, 29, 641–653. [Google Scholar] [PubMed]
- Wang, K.; Shu, C.; Zhang, J. Effective bacterial insecticidal proteins against coleopteran pests: A review. Arch. Insect Biochem. Physiol. 2019, 102, e21558. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J.Invertebr. Pathol. 2020, 107438. [Google Scholar] [CrossRef]
- Heckel, D.G. How do toxins from Bacillus thuringiensis kill insects? An evolutionary perspective. Arch. Insect Biochem. Physiol. 2020, 104, e21673. [Google Scholar] [CrossRef] [Green Version]
- Pardo-López, L.; Soberón, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Lambert, B.; Höfte, H.; Annys, K.; Jansens, S.; Soetaert, P.; Peferoen, M. Novel Bacillus thuringiensis insecticidal crystal protein with a silent activity against coleopteran larvae. Appl. Environ. Microbiol. 1992, 58, 2536–2542. [Google Scholar] [CrossRef] [Green Version]
- Carroll, J.; Convents, D.; Van Damme, J.; Boets, A.; Van Rie, J.; Ellar, D.J. Intramolecular proteolytic cleavage of Bacillus thuringiensis Cry3A delta-endotoxin may facilitate its coleopteran toxicity. J. Invertebr. Pathol. 1997, 70, 41–49. [Google Scholar] [CrossRef]
- Hey, T.D.; Larrinua, I.M.; Narva, K.E. Modified Bacillus thuringiensis cry proteins that inhibit coleopterans. US Patent No. 8,269,069, 18 September 2012. [Google Scholar]
- Yamaguchi, T.; Sahara, K.; Bando, H.; Asano, S. Discovery of a novel Bacillus thuringiensis Cry8D protein and the unique toxicity of the Cry8D-class proteins against scarab beetles. J. Invertebr. Pathol. 2008, 99, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Shizhi, H.; Changlong, S.; Chunqin, L.; Xuezhi, D.; Jie, Z. Screening of Insecticidal Activity Protein against Holotrichia parallela Adults from Bacillus thuringiensis. Chin. J. Biol. Control 2018, 34, 546–552. [Google Scholar]
- Terra, W.R.; Ferreira, C. Insect digestive enzymes: Properties, compartmentalization and function. Compbiochemphysiol B 1994, 109, 1–62. [Google Scholar] [CrossRef]
- Zhou, Z.; Yin, X.M.; Meng-Zhu, L.U.; Jiang, Z.W.; Wang, P. mBt886cry3a Gene Transformed into Chrysanthemum Dendranthemum grandiflorum ′Xiao Huang′ Resistant to The Long Horn Beetles. North. Hortic. 2009, 11, 48–50. [Google Scholar]
- Chen, J.; Dai, L.Y.; Wang, X.-P.; Tian, Y.C.; Lu, M. The cry3Aa gene of Bacillus thuringiensis Bt886 encodes a toxin against long-horned beetles. Appl. Microbiol. Biotechnol. 2005, 67, 351–356. [Google Scholar] [CrossRef]
- Guo, C.H.; Zhao, S.T.; Ma, Y.; Hu, J.J.; Han, X.J.; Chen, J.; Lu, M.Z. Bacillus thuringiensisCry3Aa fused to a cellulase-binding peptide shows increased toxicity against the longhorned beetle. Appl. Microbiol. Biotechnol. 2012, 93, 1249–1256. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Zhang, M.; Fabrick, J.A.; Wu, Y.; Gao, M.; Huang, F.; Wei, J.; Zhang, J.; Yelich, A.; Unnithan, G.C.; et al. Dual mode of action of Bt proteins: Protoxin efficacy against resistant insects. Sci. Rep. 2015, 5, 15107. [Google Scholar] [CrossRef] [Green Version]
- Peña-Cardeña, A.; Grande, R.; Sánchez, J.; Tabashnik, B.E.; Bravo, A.; Soberón, M.; Gómez, I. The C-terminal protoxin region of Bacillus thuringiensis Cry1Ab toxin has a functional role in binding to GPI-anchored receptors in the insect midgut. J. Biol. Chem. 2018, 293, 20263–20272. [Google Scholar] [CrossRef] [Green Version]
- Shu, C.; Yan, G.; Wang, R.; Zhang, J.; Feng, S.; Huang, D.; Song, F. Characterization of a novel cry8 gene specific to Melolonthidae pests: Holotrichia oblita and Holotrichia parallela. Appl. Microbiol. Biotechnol. 2009, 84, 701–707. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, G.; Tan, J.; Li, C.; Cheng, L. Cloning and characterization of a novel cry8Ab1 gene from Bacillus thuringiensis strain B-JJX with specific toxicity to scarabaeid (Coleoptera: Scarabaeidae) larvae. Microbiol. Res. 2013, 168, 512–517. [Google Scholar] [CrossRef]
- Shu, C.; Yu, H.; Wang, R.; Fen, S.; Su, X.; Huang, D.; Zhang, J.; Song, F. Characterization of two novel cry8 genes from Bacillus thuringiensis strain BT185. Curr. Microbiol. 2009, 58, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, R.; Shu, C.; Zhang, Q.; Zhao, S.; Shao, G.; Zhang, X.; Gao, J. Characterization of one novel cry8 gene from Bacillus thuringiensis strain Q52–7. World J. Microbiol. Biotechnol. 2014, 30, 3075–3080. [Google Scholar] [CrossRef]
- Sato, R.; Takeuchi, K.; Ogiwara, K.; Minami, M.; Kaji, Y.; Suzuki, N.; Hori, H.; Asano, S.; Ohba, M.; Iwahana, H. Cloning, heterologous expression, and localization of a novel crystal protein gene from Bacillus thuringiensis serovar japonensis strain buibui toxic to scarabaeid insects. Curr. Microbiol. 1994, 28, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Liu, R.; Wang, R.; Zhang, J.; Feng, S.; Huang, D.; Song, F. Improving toxicity of Bacillus thuringiensis strain contains the cry8Ca gene specific to Anomala corpulenta larvae. Curr. Microbiol. 2007, 55, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.; Yamashita, C.; Iizuka, T.; Takeuchi, K.; Yamanaka, S.; Cerf, D.; Yamamoto, T. A strain of Bacillus thuringiensis subsp. galleriae containing a novel cry8 gene highly toxic to Anomala cuprea (Coleoptera: Scarabaeidae). Biol. Control 2003, 28, 191–196. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Song, F.; Wu, J.; Feng, S.; Huang, D. Engineered Bacillus thuringiensis GO33A with broad insecticidal activity against lepidopteran and coleopteran pests. Appl. Microbiol. Biotechnol. 2006, 72, 924–930. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Boil. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]

| Sample | H. oblita | H. parallela | C. bowringi | ||
|---|---|---|---|---|---|
| Adult LC50 Values in mg/mL (Confidence Limits) | Larvae 108 CFU/g | Adult LC50 Values in mg/mL (Confidence Limits) | Larvae LC50 Values in 108 CFU/g (Confidence Limits) | Larvae LC50 Values in mg/mL (Confidence Limits) | |
| Cry8Ha1-Pro | 0.99 (0.62–2.05) | NA | 0.16 (0.00–0.57) | 218.90 (68.21–661.10) | NA |
| Cry8Ia1-Pro | NA | NA | NA | 85.03 (30.59–176.08) | NA |
| Cry8Ha1-Act | NA | NT | 0.66 (0.20–12.36) | NT | 0.02 (0.01–0.02) |
| Cry8Ia1-Act | NA | NT | NA | NT | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, C.; Yan, G.; Huang, S.; Geng, Y.; Soberón, M.; Bravo, A.; Geng, L.; Zhang, J. Characterization of Two Novel Bacillus thuringiensis Cry8 Toxins Reveal Differential Specificity of Protoxins or Activated Toxins against Chrysomeloidea Coleopteran Superfamily. Toxins 2020, 12, 642. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100642
Shu C, Yan G, Huang S, Geng Y, Soberón M, Bravo A, Geng L, Zhang J. Characterization of Two Novel Bacillus thuringiensis Cry8 Toxins Reveal Differential Specificity of Protoxins or Activated Toxins against Chrysomeloidea Coleopteran Superfamily. Toxins. 2020; 12(10):642. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100642
Chicago/Turabian StyleShu, Changlong, Guixin Yan, Shizhi Huang, Yongxin Geng, Mario Soberón, Alejandra Bravo, Lili Geng, and Jie Zhang. 2020. "Characterization of Two Novel Bacillus thuringiensis Cry8 Toxins Reveal Differential Specificity of Protoxins or Activated Toxins against Chrysomeloidea Coleopteran Superfamily" Toxins 12, no. 10: 642. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12100642







