Biological Control of Aflatoxin in Maize Grown in Serbia
Abstract
:1. Introduction
2. Results
2.1. Monitoring Deletions in the Aflatoxin Biosynthesis Isolate Mytoolbox Af01 Gene Cluster
2.2. Quality Control of Atoxigenic Product
2.3. Intensity of A. flavus Infection in Maize
2.4. AFB1 Content
2.5. Climate Conditions
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Selection of Aspergillus flavus Strains
5.2. Monitoring Deletions in the Aflatoxin Biosynthesis Isolate Mytoolbox Af01 Gene Cluster
5.3. Biocontrol Product Preparation
5.4. Quality Control of the Atoxigenic Biocontrol Product Following Cotty (pers. comm.)
5.5. Sowing Maize and Application of the Atoxigenic Isolate
5.6. Evaluation of Intensity of A. flavus Infection in Maize
5.7. Harvest and Samples Preparation
5.8. ELISA Test
5.9. Statistical Analyses
5.10. Climate Conditions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cotty, P.J.; Bayman, P.; Egel, D.S.; Elias, K.S. Agriculture, aflatoxins and Aspergillus. In The Genus Aspergillus: From Taxonomy and Genetics to Industrial Application; Powel, K.A., Renwick, A., Peverdy, J.F., Eds.; Plenum Press: New York, NY, USA, 1994; pp. 1–27. [Google Scholar]
- Bandyopadhyay, R.; Ortega-Beltran, A.; Akande, A.; Mutegi, C.; Atehnkeng, J.; Kaptoge, L.; Senghor, A.L.; Adhikari, B.N.; Cotty, P.J. Biological control of aflatoxins in Africa: Current status and potential challenges in the face of climate changes. World Mycotoxin J. 2016, 1–20. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Aflatoxins. In Monograph on the Evaluation of Carcinogenic Risks to Humans. Some Traditional Herbal Medicines, some Mycotoxins, Naphthalene and Styrene; IARC: Lyon, France, 2002; Volume 82, pp. 171–300. [Google Scholar]
- Payne, A.G.; Yu, J. Ecology, development and gene regulation in Aspergillus flavus. In Aspergillus: Molecular Biology and Genomics; Machida, M., Gomi, K., Eds.; Caister Academic Press: Norfolk, UK, 2010. [Google Scholar]
- Mehl, H.L.; Jaime, R.; Callicott, K.A.; Probst, C.; Garber, N.P.; Ortega-Beltran, A.; Grubisha, L.C.; Cotty, P.J. Aspergillus flavus diversity on crops and in the environment can be exploited to reduce aflatoxin exposure and improve health. Ann. N. Y. Acad. Sci. 2012, 1273, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.P. Breeding for resistance to aflatoxin accumulation in maize. Mycotoxin Res. 2006, 22, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J. Cottonseed losses and mycotoxins. In Compendium of Cotton Diseases; Kirkpatrick, T.L., Rothrock, C.S., Eds.; The American Phytopathological Society: Saint Paul, MN, USA, 2001; pp. 9–13. [Google Scholar]
- Cotty, P.J.; Mellon, J.E. Ecology of aflatoxin-producing fungi and biocontrol of aflatoxin contamination. Mycotoxin Res. 2006, 22, 110–117. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Leggieri, M.C.; Brera, C.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [Green Version]
- Lević, J.; Gošić-Dondo, S.; Ivanović, D.; Stanković, S.; Krnjaja, V.; Bočarov-Stančić, A.; Stepanić, A. An outbreak of Aspergillus species in response to environmental conditions in Serbia. Pestic. Phytomed. 2013, 28, 167–179. [Google Scholar] [CrossRef]
- Kos, J.; Mastilović, J.; Janić Hajnal, E.; Šarić, B. Natural occurrence of aflatoxins in maize harvested in Serbia during 2009–2012. Food Control 2013, 34, 31–34. [Google Scholar] [CrossRef]
- Cotty, P.J.; Bayman, P. Competitive exclusion of a toxigenic strain of Aspergillus flavus by an atoxigenic strain. Phytopathology 1993, 83, 1283–1287. [Google Scholar] [CrossRef]
- Cotty, P.J. Biocompetitive exclusion of toxigenic fungi. In The Mycotoxin Factbook; Barug, D., Bhatnagar, D., Van Egmond, H.P., Van der Kamp, J.W., Van Osenbruggen, W.A., Visconti, A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 179–197. [Google Scholar]
- Atehnkeng, J.; Ojiambo, P.S.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Addit. Contam. 2008, 25, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W. Biological control of aflatoxin contamination in corn using a nontoxigenic strain of Aspergillus flavus. J. Food Prot. 2009, 72, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Bandyopadhyay, R.; Price, L.E.; Cotty, P.J. Identification of atoxigenic Aspergillus flavus isolates to reduce aflatoxin contamination of maize in Kenya. Plant Dis. 2011, 95, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callicott, K.A.; Cotty, P.J. Method for monitoring deletions in the aflatoxin biosynthesis gene cluster of Aspergillus flavus with multiplex PCR. Lett. Appl. Microbiol. 2014, 60, 60–65. [Google Scholar] [CrossRef]
- Chang, P.; Horn, B.W.; Dorner, J.W. Sequence breakpoints in the aflatoxin biosynthesis gene cluster and flanking regions in nonaflatoxigenic Aspergillus flavus isolates. Fungal Genet. Biol. 2005, 42, 914–923. [Google Scholar] [CrossRef]
- Yin, Y.; Lou, T.; Yan, L.; Michailides, T.; Ma, Z. Molecular characterization of toxigenic and atoxigenic Aspergillus flavus isolates, collected from peanut fields in China. J. Appl. Microbiol. 2009, 107, 1857–1865. [Google Scholar] [CrossRef]
- Donner, M.; Atehnkeng, J.; Sikora, R.A.; Bandyopadhyay, R.; Cotty, P.J. Molecular characterization of atoxigenic strains for biological control of aflatoxins in Nigeria. Food Addit. Contam. 2010, 27, 576–590. [Google Scholar] [CrossRef]
- Adhikari, B.N.; Bandyopadhyay, R.; Cotty, P.J. Degeneration of aflatoxin gene clusters in Aspergillus flavus from Africa and North America. AMB Express 2016, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Mukanga, M.; Derera, J.; Tongoona, P.; Laing, M.D. A survey of pre-harvest ear rot diseases of maize and associated mycotoxins in south and central Zambia. Int. J. Food Microbiol. 2010, 141, 213–221. [Google Scholar] [CrossRef]
- Williams, W.P.; Ozkan, S.; Ankala, A.; Windham, G.L. Ear rot, aflatoxin accumulation, and fungal biomass in maize after inoculation with Aspergillus flavus. Field Crops Res. 2011, 120, 196–200. [Google Scholar] [CrossRef]
- Dorner, J.W.; Cole, R.J.; Wicklow, D.T. Aflatoxin reduction in corn through field application of competitive fungi. J. Food Prot. 1999, 62, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Atehnkeng, J.; Oijambo, P.S.; Cotty, P.J.; Bandyopadhyay, R. Field efficacy of a mixture of atoxigenic Aspergillus flavus Link: Fr vegetative compatibility groups in preventing aflatoxin contamination in maize (Zea mays L.). Biol. Control 2014, 72, 62–70. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Bruns, H.A.; Abel, C.A. Biocontrol of aflatoxin in corn by inoculation with non-aflatoxigenic Aspergillus flavus isolates. Biocontrol Sci. Technol. 2006, 16, 437–449. [Google Scholar] [CrossRef]
- Payne, G.A.; Cassel, D.K.; Adkins, C.R. Reduction of aflatoxin contamination in corn by irrigation and tillage. Phytopathology 1986, 76, 679–684. [Google Scholar] [CrossRef]
- Bruns, H.A. Controlling aflatoxin and fumonisin in maize by crop management. J. Toxicol. 2003, 22, 153–173. [Google Scholar] [CrossRef]
- Guo, B.; Chen, Z.; Lee, R.D.; Scully, B.T. Drought stress and preharvest aflatoxin contamination in agricultural commodity: Genetics, genomics and proteomics. J. Integr. Plant Biol. 2008, 50, 1281–1291. [Google Scholar] [CrossRef]
- Chauhan, Y.S.; Wright, G.C.; Rachaputi, N.C. Modelling climatic risks of aflatoxin contamination in maize. Aust. J. Exp. Agric. 2008, 48, 358–366. [Google Scholar] [CrossRef]
- Cotty, P.J. Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology 1989, 79, 808–814. [Google Scholar] [CrossRef] [Green Version]
- Barošević, T.; Bagi, F.; Budakov, D.; Kocsubé, S.; Varga, J.; Grahovac, M.; Stojšin, V. Molecular and morphological identification of Aspergillus species on corn seeds. In Proceedings of the III International Congress Food Technology, Quality and Safety, Novi Sad, Serbia, 25–27 October 2016; University of Novi Sad, Institute of Food Technology: Novi Sad, Serbia, 2016; pp. 365–371. [Google Scholar]
- Brody, J.R.; Kern, S.E. Sodium boric acid: A Trisfree, cooler conductive medium for DNA electrophoresis. Biotechniques 2004, 36, 214–216. [Google Scholar] [CrossRef]
- Garber, N.P.; Ortega-Beltran, A.; Barker, G.; Probst, C.; Callicott, K.A.; Jaime, R.; Mehl, H.L.; Cotty, P.J. Brief Protocols for Research on Management of Aflatoxin-Producing Fungi, 1st ed.; School of Plant Sciences, The University of Arizona: Tucson, AZ, USA, 2012; pp. 95–100. [Google Scholar]
- Cotty, P.J.; University of Arizona, Tucson, AZ, USA. Personal communication, 2016.
- Reid, L.M.; Hamilton, R.E.; Mather, D.E. Screening Maize for Resistance to Gibberella Ear Rot; Technical Bulletin; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 1996; p. 62. [Google Scholar]
- European Commission No 401/2006 of 23 February 2006. Laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union L. 2006, 70, 12–34. [Google Scholar]
- Kebede, H.; Abbas, H.K.; Fisher, D.K.; Bellaloui, N. Relationship between aflatoxin contamination and physiological responses of corn plants under drought and heat stress. Toxins 2012, 4, 1385–1403. [Google Scholar] [CrossRef] [PubMed]
- Republic Hydrometeorological Service of Serbia. Multiannual Average of Meteorological Parameters (1981–2010). Available online: http://www.hidmet.gov.rs/ciril/meteorologija/klimatologija_srednjaci.php (accessed on 5 November 2019).



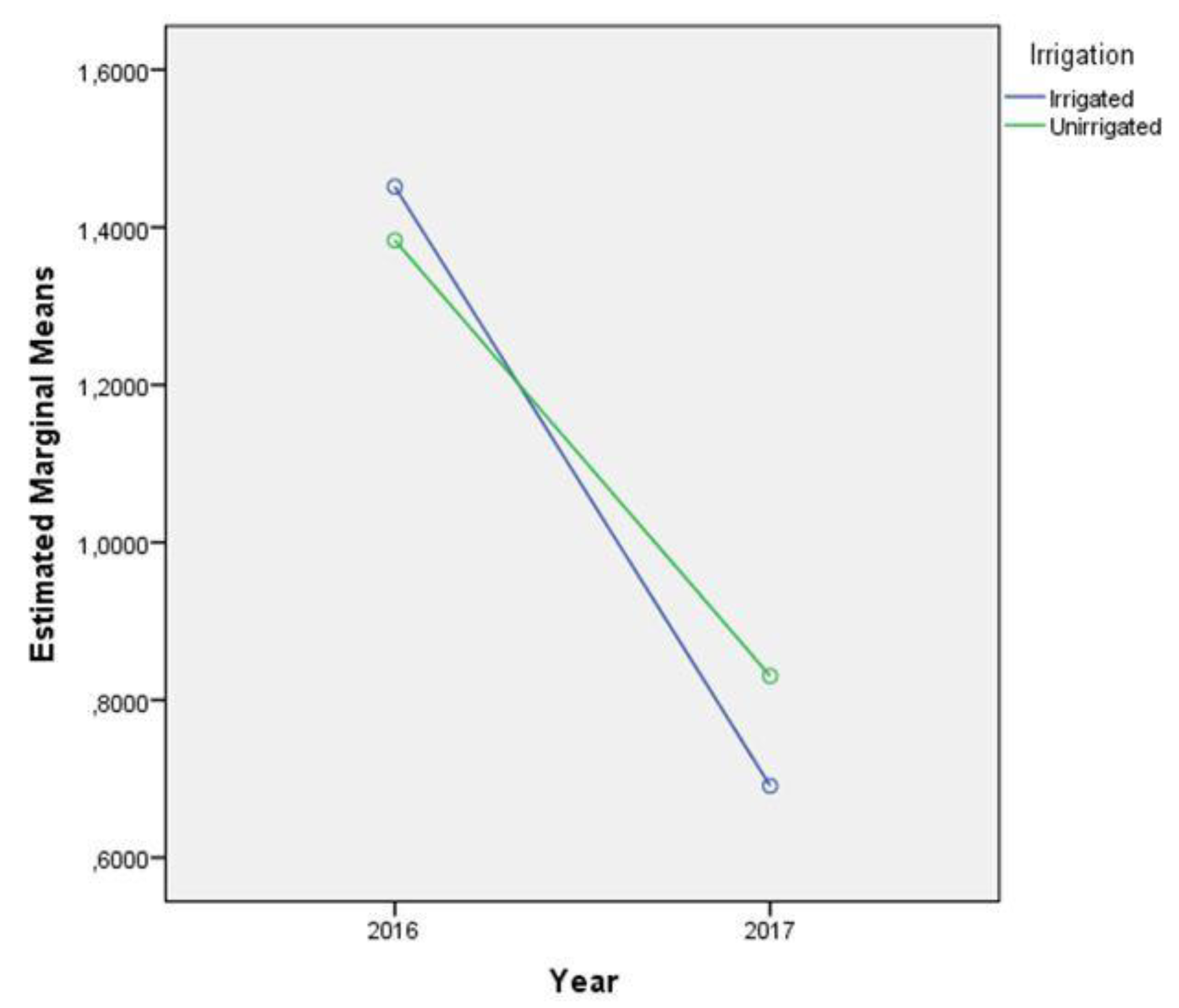


| Effect | N | Mean | Standard Deviation | t-Test for Equality of Means | ||
|---|---|---|---|---|---|---|
| t | df | Significance Probability (2-Tailed) | ||||
| Biocontrol | ||||||
| Treated | 32 | 2.31 | 6.713 | −3.858 | 62 | 0.000 |
| Untreated | 32 | 8.68 | 6.483 | |||
| Irrigation | ||||||
| Irrigated | 32 | 5.75 | 8.215 | 0.279 | 62 | 0.782 |
| Unirrigated | 32 | 5.24 | 6.355 | |||
| Effect | Type III Sum of Squares | df | Mean Square | F | Significance Probability | Partial Eta Squared |
|---|---|---|---|---|---|---|
| Intercept | 75.913 | 1 | 75.913 | 98.064 | 0.000 | 0.778 |
| Biocontrol | 28.057 | 1 | 28.057 | 36.244 | 0.000 | 0.564 |
| Irrigation | 0.020 | 1 | 0.020 | 0.026 | 0.873 | 0.001 |
| Biocontrol*Irrigation | 0.342 | 1 | 0.342 | 0.442 | 0.512 | 0.016 |
| Error | 21.675 | 28 | 0.774 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savić, Z.; Dudaš, T.; Loc, M.; Grahovac, M.; Budakov, D.; Jajić, I.; Krstović, S.; Barošević, T.; Krska, R.; Sulyok, M.; et al. Biological Control of Aflatoxin in Maize Grown in Serbia. Toxins 2020, 12, 162. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12030162
Savić Z, Dudaš T, Loc M, Grahovac M, Budakov D, Jajić I, Krstović S, Barošević T, Krska R, Sulyok M, et al. Biological Control of Aflatoxin in Maize Grown in Serbia. Toxins. 2020; 12(3):162. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12030162
Chicago/Turabian StyleSavić, Zagorka, Tatjana Dudaš, Marta Loc, Mila Grahovac, Dragana Budakov, Igor Jajić, Saša Krstović, Tijana Barošević, Rudolf Krska, Michael Sulyok, and et al. 2020. "Biological Control of Aflatoxin in Maize Grown in Serbia" Toxins 12, no. 3: 162. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins12030162









