Evaluation of Cyanobacterial Bloom from Lake Taihu as a Protein Substitute in Fish Diet—A Case Study on Tilapia
Abstract
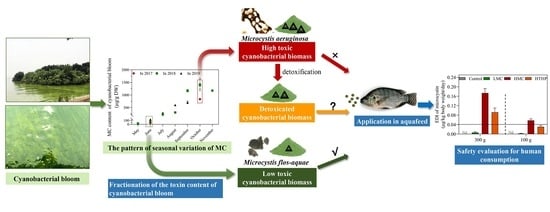
:1. Introduction
2. Results
2.1. The Effects of Dietary Cyanobacteria on Growth of Tilapia
2.2. Safety Evaluation of Tilapia Based on TDI in Different Treatments
2.3. Temporal Changes in MC Content from 2017 to 2019 in Lake Taihu
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Tilapia, Cyanobacterial Biomass, and Experimental Diets
5.2. Experimental Procedure and Sample Collection
5.3. Microcystin Analysis
5.4. Risk Assessment
5.5. Enzymatic Analysis
5.6. Calculations and Statistical Analysis
5.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sha, J.; Xiong, H.; Li, C.; Lu, Z.; Zhang, J.; Zhong, H.; Zhang, W.; Yan, B. Harmful algal blooms and their eco-environmental indication. Chemosphere 2021, 274, 129912. [Google Scholar] [CrossRef]
- Hu, L.; Shan, K.; Huang, L.; Li, Y.; Zhao, L.; Zhou, Q.; Song, L. Environmental factors associated with cyanobacterial assemblages in a mesotrophic subtropical plateau lake: A focus on bloom toxicity. Sci. Total Environ. 2021, 777, 146052. [Google Scholar] [CrossRef]
- Teta, R.; Sala, G.; Esposito, G.; Stornaiuolo, M.; Scarpato, S.; Casazza, M.; Anastasio, A.; Lega, M.; Costantino, V. Monitoring cyanobacterial blooms during the COVID-19 pandemic in Campania, Italy: The case of Lake Avernus. Toxins 2021, 13, 471. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Genet. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Arhonditsis, G.B.; Gao, J.; Chen, Q.; Peng, J. The magnitude and drivers of harmful algal blooms in China’s lakes and reservoirs: A national-scale characterization. Water Res. 2020, 181, 115902. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.K.; Kottuparambil, S.; Moh, S.H.; Lee, T.K.; Kim, Y.-J.; Rhee, J.-S.; Choi, E.-M.; Kim, B.H.; Sanghyun, M.; Yarish, C.; et al. Potential applications of nuisance microalgae blooms. J. Appl. Phycol. 2014, 27, 1223–1234. [Google Scholar] [CrossRef]
- Shen, Q.; Li, D.; Li, D.; Liu, Y.; Li, J.; Li, S. Study on the safe disposal and resource utilization of cyanobacterial bloom biomass in Dianchi Lake, China. J. Appl. Phycol. 2019, 32, 1201–1213. [Google Scholar] [CrossRef]
- Paerl, H. Mitigating toxic planktonic cyanobacterial blooms in aquatic ecosystems facing increasing anthropogenic and climatic pressures. Toxins 2018, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Mucci, M.; Guedes, I.A.; Faassen, E.J.; Lürling, M. Chitosan as a coagulant to remove cyanobacteria can cause microcystin release. Toxins 2020, 12, 711. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jia, Y.; Liu, A.; Zhou, Q.; Song, L. Simultaneous elimination of cyanotoxins and PCBs via mechanical collection of cyanobacterial blooms: An application of green-bioadsorption concept. J. Environ. Sci. 2017, 57, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jia, Y.; Li, E.; Zhao, S.; Zhou, Q.; Liu, L.; Song, L. Soil-based treatments of mechanically collected cyanobacterial blooms from Lake Taihu: Efficiencies and potential risks. Environ. Sci. Technol. 2012, 46, 13370–13376. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.-C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, J.; Zhou, Q.; Liu, G.; Wang, T. Harmless disposal and resource utilization of wastes from the lake in China: Dewatering, composting and safety evaluation of fertilizer. Algal Res. 2019, 43, 101623. [Google Scholar] [CrossRef]
- Qin, B.; Paerl, H.W.; Brookes, J.; Liu, J.; Jeppesen, E.; Zhu, G.; Zhang, Y.; Xu, H.; Shi, K.; Deng, J. Why Lake Taihu continues to be plagued with cyanobacterial blooms through 10 years (2007–2017) efforts. Sci. Bull. 2019, 64, 354–356. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Hu, M.; Bian, B.; Yang, Z.; Yang, W.; Zhang, L. Full-scale thermophilic aerobic co-composting of blue-green algae sludge with livestock faeces and straw. Sci. Total Environ. 2021, 753, 142079. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, W.; Hu, S.; Feng, G.; Xue, Z.; Chen, H. Hydrothermal pretreatment of salvaged cyanobacteria and use of pretreated medium for cultivating Scenedesmus obliquus. Bioresour. Technol. 2019, 294, 122120. [Google Scholar] [CrossRef]
- Wei, L.; Li, X.; Yi, J.; Yang, Z.; Wang, Q.; Ma, W. A simple approach for the efficient production of hydrogen from Taihu Lake Microcystis spp. blooms. Bioresour. Technol. 2013, 139, 136–140. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, W.; Zhang, Y.; Qiao, Q.; Zhang, X. Are fish fed with cyanobacteria safe, nutritious and delicious? A laboratory study. Sci. Rep. 2015, 5, 15166. [Google Scholar] [CrossRef] [Green Version]
- Servaites, J.C.; Faeth, J.L.; Sidhu, S.S. A dye binding method for measurement of total protein in microalgae. Anal. Biochem. 2012, 421, 75–80. [Google Scholar] [CrossRef]
- Bickel, H.; Lyck, S.; Utkilen, H. Energy state and toxin content—Experiments on Microcystis aeruginosa (Chroococcales, Cyanophyta). Phycologia 2019, 39, 212–218. [Google Scholar] [CrossRef]
- Acuña, S.; Baxa, D.; Teh, S. Sublethal dietary effects of microcystin producing Microcystis on threadfin shad, Dorosoma petenense. Toxicon 2012, 60, 1191–1202. [Google Scholar] [CrossRef]
- Acuña, S.; Deng, D.-F.; Lehman, P.; Teh, S. Sublethal dietary effects of Microcystis on Sacramento splittail, Pogonichthys macrolepidotus. Aquat. Toxicol. 2012, 110, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xie, S.; Zhu, X.; Yang, Y.; Gan, N.; Song, L. Effect of dietary cyanobacteria on growth and accumulation of microcystins in Nile tilapia (Oreochromis niloticus). Aquaculture 2006, 261, 960–966. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lalić, D.; Savić, G.B.; Tokodi, N.; Backović, D.D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Dong, G.; Xie, S.; Zhu, X.; Han, D.; Yang, Y.; Song, L.; Gan, L.; Chen, W. Responses of yellow catfish (Pelteobagrus fulvidraco Richardson) exposed to dietary cyanobacteria and subsequent recovery. Toxicon 2012, 60, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhu, X.; Han, N.; Yang, Y.; Song, L.; Xie, S. Effects of dietary cyanobacteria of two different sources on growth and recovery of hybrid tilapia (Oreochromis niloticus × O. aureus). Toxicon 2009, 54, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhu, X.; Han, D.; Yang, Y.; Song, L.; Xie, S. Response and recovery of hybrid sturgeon from subchronic oral administration of cyanobacteria. Environ. Toxicol. 2011, 26, 161–170. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Liu, W.; Xia, H.; Li, J.; Zhang, X. Effects of toxic cyanobacteria and ammonia on flesh quality of blunt snout bream (Megalobrama amblycephala). J. Sci. Food Agric. 2017, 97, 1200–1206. [Google Scholar] [CrossRef]
- Hu, L.; Shan, K.; Lin, L.; Shen, W.; Huang, L.; Gan, N.; Song, L. Multi-year assessment of toxic genotypes and Microcystin concentration in Northern Lake Taihu, China. Toxins 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotto, A.M.; Satchwell, M.F.; Berry, D.L.; Gobler, C.J.; Boyer, G.L. Spatial and temporal diversity of microcystins and microcystin-producing genotypes in Oneida Lake, NY. Harmful Algae 2008, 7, 671–681. [Google Scholar] [CrossRef]
- Yoshida, M.; Yoshida, T.; Takashima, Y.; Hosoda, N.; Hiroishi, S. Dynamics of microcystin-producing and non-microcystin-producing Microcystis populations is correlated with nitrate concentration in a Japanese lake. FEMS Microbiol. Lett. 2007, 266, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wu, Y.; Song, L.; Gan, N. Ecological dynamics of toxic Microcystis spp. and microcystin-degrading bacteria in Dianchi Lake, China. Appl. Environ. Microbiol. 2014, 80, 1874–1881. [Google Scholar] [CrossRef] [Green Version]
- El Asely, A.; Amin, A.; El-Naby, A.S.A.; Samir, F.; El-Ashram, A.; Dawood, M. Ziziphus mauritiana supplementation of Nile tilapia (Oreochromis niloticus) diet for improvement of immune response to Aeromonas hydrophila infection. Fish Physiol. Biochem. 2020, 46, 1561–1575. [Google Scholar] [CrossRef]
- FAO. FAO Global Aquaculture Production 1950–2019 (Online Query). 2021. Available online: http://www.fao.org/fishery/statistics/global-aquaculture-production/query/en (accessed on 17 March 2021).
- Teuling, E.; Schrama, J.; Gruppen, H.; Wierenga, P.A. Effect of cell wall characteristics on algae nutrient digestibility in Nile tilapia (Oreochromis niloticus) and African catfish (Clarus gariepinus). Aquaculture 2017, 479, 490–500. [Google Scholar] [CrossRef]
- World Health Organization. Cyanobacterial toxins: Microcystins. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ziková, A.; Trubiroha, A.; Wiegand, C.; Wuertz, S.; Rennert, B.; Pflugmacher, S.; Kopp, R.; Mareš, J.; Kloas, W. Impact of microcystin containing diets on physiological performance of Nile tilapia (Oreochromis niloticus) concerning stress and growth. Environ. Toxicol. Chem. 2010, 29, 561–568. [Google Scholar] [CrossRef]
- Torres, G.S.; Silva, L.; Rangel, L.M.; Attayde, J.; Huszar, V.L.M. Cyanobacteria are controlled by omnivorous filter-feeding fish (Nile tilapia) in a tropical eutrophic reservoir. Hydrobiologia 2015, 765, 115–129. [Google Scholar] [CrossRef]
- Lu, K.; Jin, C.; Dong, S.; Gu, B.; Bowen, S.H. Feeding and control of blue-green algal blooms by tilapia (Oreochromis Niloticus). Hydrobiologia 2006, 568, 111–120. [Google Scholar] [CrossRef]
- Pal, M.; Yesankar, P.J.; Dwivedi, A.; Qureshi, A. Biotic control of harmful algal blooms (HABs): A brief review. J. Environ. Manag. 2020, 268, 110687. [Google Scholar] [CrossRef] [PubMed]
- Deblois, C.P.; Giani, A.; Bird, D.F. Experimental model of microcystin accumulation in the liver of Oreochromis niloticus exposed subchronically to a toxic bloom of Microcystis sp. Aquat. Toxicol. 2011, 103, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xie, S.; Zhu, X.; Yang, Y.; Gan, L.; Song, L. Effect of inclusion of blue-green algae meal on growth and accumulation of microcystins in gibel carp (Carassius auratus gibelio). J. Appl. Ichthyol. 2006, 22, 72–78. [Google Scholar] [CrossRef]
- Bureau of Environmental Health. Bureau of Environmental Health Blue-Green Algae/Cyanobacteria Harmful Algal Blooms (HABs) Physician Reference. 2010. Available online: https://www.hamiltoncountyhealth.org/wp-content/uploads/HAB-Provider (accessed on 20 May 2021).
- Centers for Disease Control. Centers for Disease Control Veterinarian Reference: Cyanobacteria Blooms. 2016. Available online: https://www.cdc.gov/habs/pdf/habsveterinarian (accessed on 21 May 2021).
- Carbis, C.R.; Rawlin, G.T.; Grant, P.; Mitchell, G.F.; Anderson, J.W.; McCauley, I. A study of feral carp, Cyprinus carpio L., exposed to Microcystis aeruginosa at Lake Mokoan, Australia, and possible implications for fish health. J. Fish Dis. 1997, 20, 81–91. [Google Scholar] [CrossRef]
- Palíková, M.; Kovářů, F.; Navrátil, S.; Kubala, L.; Pešák, S.; Vajcová, V. The effect of pure microcystin LR and biomass of blue-green algae on selected immunological indices of carp (Cyprinus carpio L.) and Silver carp (Hypophthalmichthys molitrix Val.). Acta Vet. Brno. 1998, 67, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Navrátil, S.; Palíková, M.; Vajcová, V. The effects of pure microcystin LR and biomass of blue-green algae on blood indices of carp (Cyprinus carpio L.). Acta Vet. Brno. 1998, 67, 273. [Google Scholar] [CrossRef]
- Le Manach, S.; Sotton, B.; Huet, H.; Duval, C.; Paris, A.; Marie, A.; Yépremian, C.; Catherine, A.; Mathéron, L.; Vinh, J.; et al. Physiological effects caused by microcystin-producing and non-microcystin producing Microcystis aeruginosa on medaka fish: A proteomic and metabolomic study on liver. Environ. Pollut. 2018, 234, 523–537. [Google Scholar] [CrossRef]
- Dao, T.-S.; Ortiz-Rodríguez, R.; Do-Hong, L.-C.; Wiegand, C. Non-microcystin and non-cylindrospermopsin producing cyanobacteria affect the biochemical responses and behavior of Daphnia magna. Int. Rev. Hydrobiol. 2013, 98, 235–244. [Google Scholar] [CrossRef]
- Zewde, T.W.; Johansen, J.A.; Kifle, D.; Demissie, T.B.; Hansen, J.H.; Tadesse, Z. Concentrations of microcystins in the muscle and liver tissues of fish species from Koka reservoir, Ethiopia: A potential threat to public health. Toxicon 2018, 153, 85–95. [Google Scholar] [CrossRef]
- Chia, M.A.; Abdulwahab, R.; Ameh, I.; Balogun, J.K.; Auta, J. Farmed tilapia as an exposure route to microcystins in Zaria-Nigeria: A seasonal investigation. Environ. Pollut. 2021, 271, 116366. [Google Scholar] [CrossRef]
- Zuo, J.; Hu, L.; Shen, W.; Zeng, J.; Li, L.; Song, L.; Gan, N. The involvement of alpha-proteobacteria Phenylobacterium in maintaining the dominance of toxic Microcystis blooms in Lake Taihu, China. Environ. Microbiol. 2021, 23, 1066–1078. [Google Scholar] [CrossRef]
- Ninio, S.; Lupu, A.; Viner-Mozzini, Y.; Zohary, T.; Sukenik, A. Multiannual variations in Microcystis bloom episodes—Temperature drives shift in species composition. Harmful Algae 2020, 92, 101710. [Google Scholar] [CrossRef]
- Song, L.; Chen, W.; Peng, L.; Wan, N.; Gan, N.; Zhang, X. Distribution and bioaccumulation of microcystins in water columns: A systematic investigation into the environmental fate and the risks associated with microcystins in Meiliang Bay, Lake Taihu. Water Res. 2007, 41, 2853–2864. [Google Scholar] [CrossRef] [PubMed]





| Ingredients | Control | LMC | HMC | HTHP |
|---|---|---|---|---|
| Fish meal | 330 | 220 | 220 | 220 |
| Soybean meal (Oil-extracted) | 120 | 80 | 80 | 80 |
| Rapeseed meal | 120 | 80 | 80 | 80 |
| Blood-meal | 20 | 20 | 20 | 20 |
| Starch | 190 | 210 | 210 | 210 |
| LMC | 0 | 185 | 0 | 0 |
| HMC | 0 | 0 | 188 | 0 |
| HTHP | 0 | 0 | 0 | 188 |
| Yeast food attractant | 10 | 10 | 10 | 10 |
| Mineral premix a | 50 | 50 | 50 | 50 |
| Vitamin premix b | 5 | 5 | 5 | 5 |
| Fish oil | 23 | 29 | 28.5 | 28.5 |
| Soybean oil | 23 | 29 | 28.5 | 28.5 |
| Cellulose | 109 | 82 | 80 | 80 |
| Chemical composition | ||||
| Moisture | 68 | 76 | 65 | 70 |
| Crude protein | 363 | 362 | 369 | 365 |
| Crude lipid | 85 | 85 | 83 | 84 |
| Energy (MJ/Kg DM) | 169 | 167 | 168 | 167 |
| Microcystin content (µg/g DW) | 0.00 | 3.29 | 35.3 | 26.2 |
| Methods | Conditions |
|---|---|
| Replication | 3 tanks |
| Density (tail/per tank) | 30 |
| Temperature | 25–28 °C |
| Light period | 8:00–20:00 |
| Water-dissolved oxygen | >7.4 mg/L |
| Ammonia-N | <0.5 mg/L |
| Feeding practice | By hand to apparent satiation twice daily 9:00–10:00, 15:00–16:00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Y.; Li, Y.; Guo, W.; Liu, J.; Yang, C.; Li, L.; Liu, H.; Song, L. Evaluation of Cyanobacterial Bloom from Lake Taihu as a Protein Substitute in Fish Diet—A Case Study on Tilapia. Toxins 2021, 13, 735. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13100735
Huo Y, Li Y, Guo W, Liu J, Yang C, Li L, Liu H, Song L. Evaluation of Cyanobacterial Bloom from Lake Taihu as a Protein Substitute in Fish Diet—A Case Study on Tilapia. Toxins. 2021; 13(10):735. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13100735
Chicago/Turabian StyleHuo, Yan, Yuanze Li, Wei Guo, Jin Liu, Cuiping Yang, Lin Li, Haokun Liu, and Lirong Song. 2021. "Evaluation of Cyanobacterial Bloom from Lake Taihu as a Protein Substitute in Fish Diet—A Case Study on Tilapia" Toxins 13, no. 10: 735. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13100735