Feed-Based Multi-Mycotoxin Occurrence in Smallholder Dairy Farming Systems of South Africa: The Case of Limpopo and Free State
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Performance Characteristics
2.2. Mycotoxin Occurrence in Dairy Feeds
2.2.1. Regulated Mycotoxins: A South African and European Commission Perspective
2.2.2. Non-Regulated Mycotoxins
2.3. Comparisons for Mycotoxin Variability
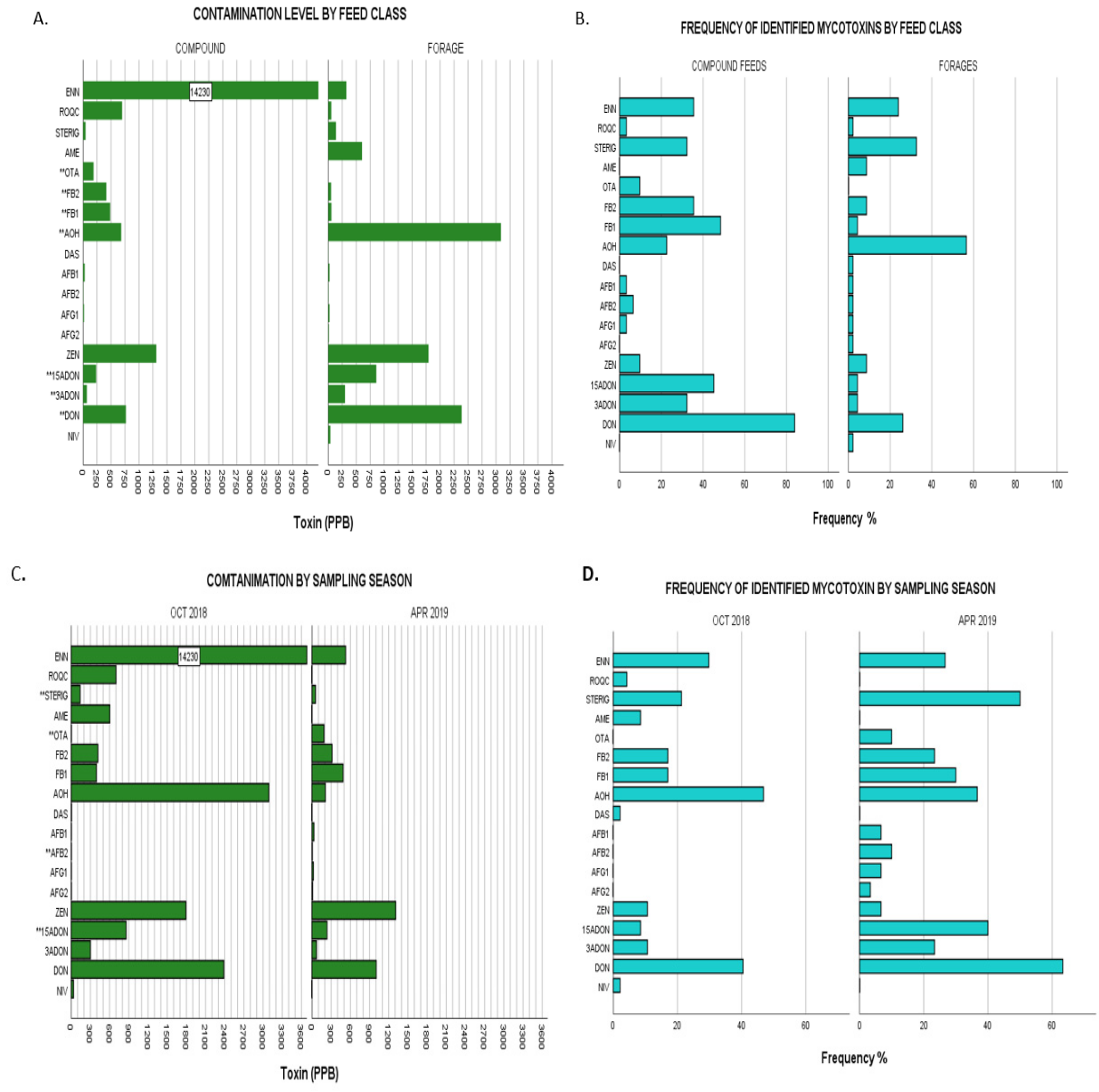
2.3.1. Comparison by Feed Class
2.3.2. Comparison by Season Sampled
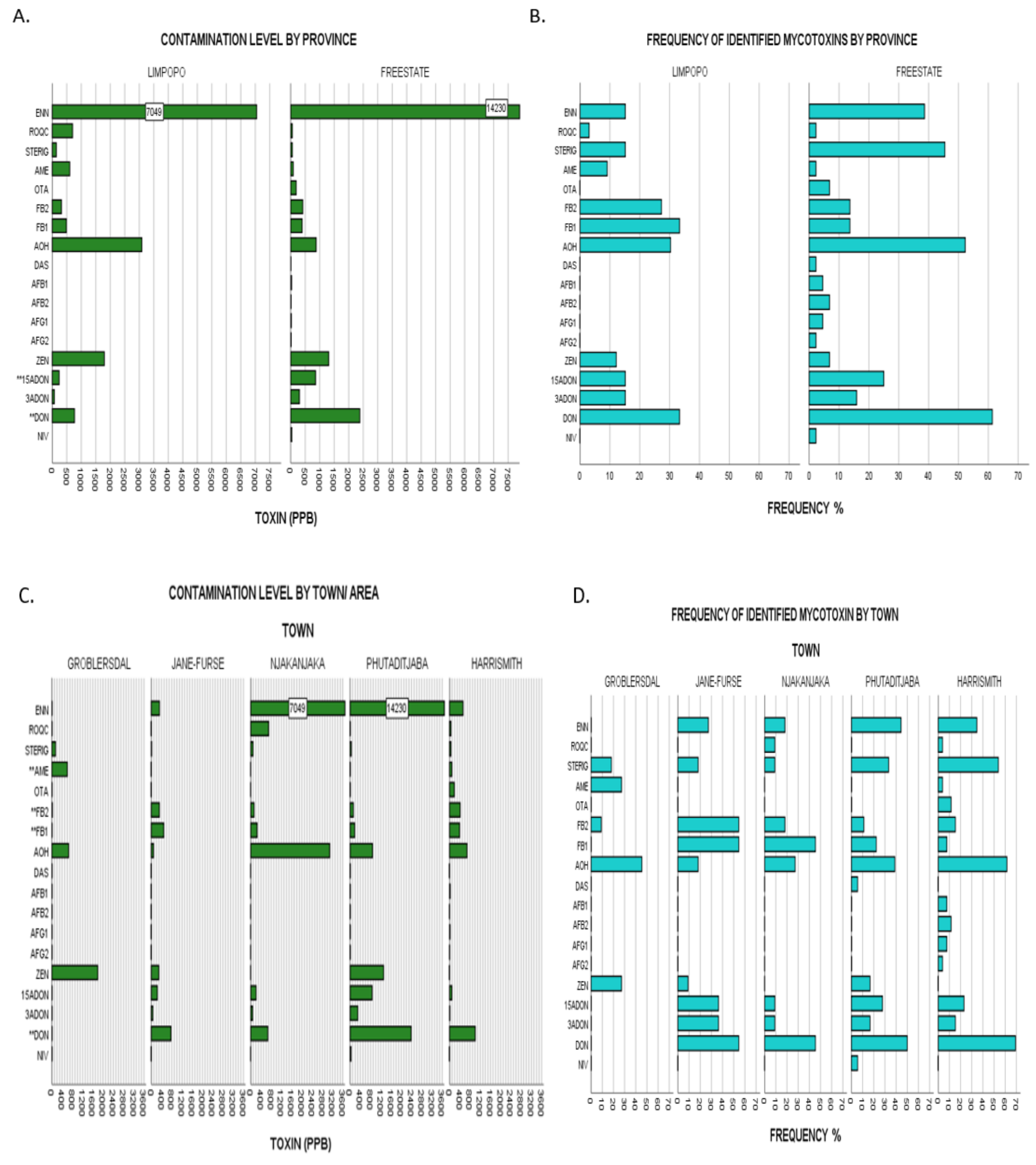
2.3.3. Comparison by Study Regions/Provinces
2.3.4. Comparison by Town Sampled
2.4. Mycotoxin Co-Occurrence
3. Conclusions
4. Materials and Methods
4.1. Study Areas and Selection Criteria
4.2. Mycotoxin Analysis
4.2.1. Chemicals and Reagents
- Legislation mix: AFB1, AFB2, AFG1 and AFG2 at concentrations of ±2 ng/µL each, OTA at ±5 ng/µL, ZEN, HT-2 and T-2 at ±10 ng/µL each and DON, FB1, FB2 at ±40 ng/µL each.
- Not in legislation mix: NIV, FUS-X and AME at individual concentrations of ±20 ng/µL; NEO and AOH at ±10 ng/µL each; 3-ADON and STERIG at ±5 ng/µL each; alongside FB3, DAS, ROQ-C and 15-ADON at concentrations of ±25 ng/µL, ±0.5 ng/µL, ±1 ng/µL and ±2.5 ng/µL, respectively.
4.2.2. Sample Preparation, Extraction and Clean-Up
4.2.3. UHPLC–MS/MS Analysis
4.2.4. Method Performance
- A minimum of two selected fragment ions (three identification points, where one fragment ion = 1.5 points).
- A signal-to-noise ratio >3 for both fragments.
- A relative retention time of ± 2.5% with regards to the IS.
- The ratio of the relative intensity of ions and spikes of similar concentrations must be comparable and range within acceptable limits (deduction not shown). Relative intensity is expressed as a percentage of intensity of the most abundant ion.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Grouping Variable: Feed Class | Grouping Variable: Season | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall Mean a (µg/kg) | Group (n) | Mean a (µg/kg) | Kruskal–Wallis H χ2 | Asymptotic. Sig. p | Mean Rank | Group (n) | Mean a (µg/kg) | Kruskal–Wallis H χ2 | Asymptotic. Sig. p | Mean Rank | |
| NIV | 0.48 | Comp (31) | nd | 0.674 | 0.412 | - | O2018 (47) | 0.79 | 0.638 | 0.424 | - |
| For (46) | 0.80 | A2019 (30) | nd | ||||||||
| DON | 235.77 | Comp (31) | 346.29 a | 20.037 | 0.000008 | 51.97 | O2018 (47) | 207.88 | 3.25 | 0.071 | - |
| For (46) | 161.29 b | 30.26 | A2019 (30) | 279.47 | |||||||
| 3-ADON | 8.65 | Comp (31) | 10.56 a | 10.085 | 0.001 | 45.23 | O2018 (47) | 9.17 | 2.12 | 0.145 | - |
| For (46) | 7.35 b | 34.8 | A2019 (30) | 7.82 | |||||||
| 15-ADON | 35.24 | Comp (31) | 55.36 a | 17.304 | 0.000032 | 48.16 | O2018 (47) | 27.07 b | 9.844 | 0.002 | 34.47 |
| For (46) | 21.68b | 32.83 | A2019 (30) | 48.05 a | 46.1 | ||||||
| ZEN | 60.55 | Comp (31) | 61.56 | 0.016 | 0.901 | - | O2018 (47) | 65.07 | 0.344 | 0.558 | - |
| For (46) | 59.86 | A2019 (30) | 53.46 | ||||||||
| AFG2 | 0.14 | Comp (31) | nd | 0.67 | 0.412 | - | O2018 (47) | nd | 1.567 | 0.211 | - |
| For (46) | 0.24 | A2019 (30) | 0.37 | ||||||||
| AFG1 | 0.52 | Comp (31) | 0.55 | 0.07 | 0.792 | - | O2018 (47) | nd | 3.175 | 0.075 | - |
| For (46) | 0.50 | A2019 (30) | 1.34 | ||||||||
| AFB2 | 0.17 | Comp (31) | 0.21 | 0.84 | 0.361 | - | O2018 (47) | nd b | 4.825 | 0.028 | 37.5 |
| For (46) | 0.15 | A2019 (30) | 0.44 a | 41.35 | |||||||
| AFB1 | 0.68 | Comp (31) | 0.97 | 0.09 | 0.763 | - | O2018 (47) | nd | 3.175 | 0.075 | - |
| For (46) | 0.48 | A2019 (30) | 1.74 | ||||||||
| DAS | 0.04 | Comp (31) | nd | 0.674 | 0.412 | - | O2018 (47) | 0.07 | 0.638 | 0.424 | - |
| For (46) | 0.07 | A2019 (30) | nd | ||||||||
| AOH | 119.68 | Comp (31) | 40.95 b | 8.355 | 0.004 | 30.9 | O2018 (47) | 172.67 | 1.638 | 0.201 | - |
| For (46) | 172.73 a | 44.46 | A2019 (30) | 36.65 | |||||||
| FB1 | 41.91 | Comp (31) | 101.05 a | 22.028 | 0.000003 | 49.58 | O2018 (47) | 23.75 | 2.483 | 0.115 | - |
| For (46) | 2.06b | 31.87 | A2019 (30) | 70.37 | |||||||
| FB2 | 25.79 | Comp (31) | 59.89 a | 10.24 | 0.001 | 45.87 | O2018 (47) | 19.45 | 0.653 | 0.419 | - |
| For (46) | 2.81 b | 34.37 | A2019 (30) | 35.72 | |||||||
| OTA | 3.33 | Comp (31) | 8.28 a | 4.570 | 0.033 | 41.23 | O2018 (47) | nd b | 4.825 | 0.028 | 37.5 |
| For (46) | nd b | 37.5 | A2019 (30) | 8.56 a | 41.35 | ||||||
| AME | 11.91 | Comp (31) | nd | 2.804 | 0.094 | - | O2018 (47) | 19.51 | 2.656 | 0.103 | - |
| For (46) | 19.93 | A2019 (30) | nd | ||||||||
| STERIG | 8.37 | Comp (31) | 5.53 | 0.072 | 0.788 | - | O2018 (47) | 8.05 b | 4.45 | 0.035 | 35.43 |
| For (46) | 10.29 | A2019 (30) | 8.87 a | 44.6 | |||||||
| ROQ-C | 9.80 | Comp (31) | 22.58 | 0.091 | 0.763 | - | O2018 (47) | 16.05 | 1.293 | 0.255 | - |
| For (46) | 1.18 | A2019 (30) | nd | ||||||||
| ENN B | 341.45 | Comp (31) | 814.88 | 2.825 | 0.093 | - | O2018 (47) | 531.01 | 0.317 | 0.573 | - |
| For (46) | 22.39 | A2019 (30) | 44.46 | ||||||||
| Grouping Variable: Province | Grouping Variable: Town | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group (n) | Mean A (µg/kg) | K-Wallis H χ2 | Asympt. Sig. p | Mean Rank | Group (n) | Mean A (µg/kg) | K-Wallis H χ2 | Asympt Sig. p | Mean Rank | Sig. Pairwise Comparison (sig B) | |
| NIV | Limp (33) FreeS (44) | Nd 0.84 | 0.75 | 0.386 | - | Grob (11) Jane-F (11) Njhak (11) Phut (18) Harri (26) | nd nd nd 2.05 nd | 3.278 | 0.512 | - | n/a |
| DON | Limp (33) FreeS (44) | 175.82 280.73 | 2.32 | 0.128 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd 293.84 233.63 323.43 251.17 | 11.208 | 0.024 | 20.00 44.45 40.00 40.00 43.62 | Grobl-Harri (0.017) |
| 3-ADON | Limp (33) FreeS (44) | 6.66 10.14 | 0.00026 | 0.987 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd 13.61 6.37 21.62 2.18 | 5.857 | 0.210 | - | n/a |
| 15-ADON | Limp (33) FreeS (44) | 29.59 39.48 | 0.55 | 0.459 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd 69.62 19.15 78.54 12.44 | 6.589 | 0.159 | - | n/a |
| ZEN | Limp (33) FreeS (44) | 80.28 45.75 | 0.60 | 0.439 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | 214.12 26.72 nd 111.83 nd | 9.365 | 0.053 | - | n/a |
| AFG2 | Limp (33) FreeS (44) | nd 0.25 | 0.75 | 0.386 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd nd nd nd 0.43 | 1.962 | 0.743 | - | n/a |
| AFG1 | Limp (33) FreeS (44) | nd 0.92 | 1.52 | 0.218 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd nd nd nd 1.55 | 3.975 | 0.409 | - | n/a |
| AFB2 | Limp (33) FreeS (44) | nd 0.30 | 2.31 | 0.129 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd nd nd nd 0.51 | 6.041 | 0.196 | - | n/a |
| AFB1 | Limp (33) FreeS (44) | nd 1.18 | 1.52 | 0.218 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd nd nd nd 2.00 | 3.975 | 0.409 | - | n/a |
| DAS | Limp (33) FreeS (44) | nd 0.08 | 0.75 | 0.386 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd nd nd 0.19 nd | 3.278 | 0.512 | - | n/a |
| AOH | Limp (33) FreeS (44) | 170.29 81.71 | 1.65 | 0.199 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | 119.28 10.43 381.17 81.41 81.92 | 5.808 | 0.214 | - | n/a |
| FB1 | Limp (33) FreeS (44) | 71.63 a 19.63 b | 4.83 | 0.028 | 43.70 35.48 | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd 145.08 69.82 21.87 18.07 | 17.188 | 0.002 | 30.50 52.95 47.64 38.22 33.58 | Harri-JaneF (0.009) Grobl-JaneF (0.012) |
| FB2 | Limp (33) FreeS (44) | 38.21 16.47 | 2.59 | 0.108 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | 1.92 93.18 19.53 11.11 20.19 | 12.221 | 0.016 | 34.36 53.77 38.68 35.72 37.12 | Phut-JaneF (0.023) Harri-JaneF (0.028) Grobl-JaneF (0.033) |
| OTA | Limp (33) FreeS (44) | nd 5.83 | 2.31 | 0.129 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd nd nd nd 9.87 | 6.041 | 0.196 | - | n/a |
| AME | Limp (33) FreeS (44) | 25.10 2.02 | 1.79 | 0.181 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | 75.29 nd nd nd 3.41 | 13.114 | 0.011 | 47.55 37.00 37.00 37.00 38.46 | Phut-Grobl (0.014) Harri-Grobl (0.033) Njhak-Grobl (0.040) JaneF-Grobl (0.040) |
| STERIG | Limp (33) FreeS (44) | 8.08 b 8.59 a | 6.34 | 0.012 | 32.83 43.63 | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | 14.14 1.93 8.15 8.34 8.77 | 7.753 | 0.101 | - | n/a |
| ROQ C | Limp (33) FreeS (44) | 21.21 1.24 | 0.50 | 0.823 | - | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd nd 63.63 nd 2.09 | 3.074 | 0.546 | - | n/a |
| ENN B | Limp (33) FreeS (44) | 241.83 b 416.16 a | 4.27 | 0.039 | 34.15 42.64 | Grobl (11) JaneF (11) Njhak (11) Phut (18) Harri (26) | nd 42.94 682.55 958.62 40.62 | 7.742 | 0.101 | - | n/a |

References
- Muntswu, A.E.; Chitura, T.; Abin, S.A.; Banga, C.B. Characterization of emerging and smallholder dairy production systems in South Africa. In Proceedings of the 50th Annual Congress of the SA Society for Animal Science, Port Elizabeth, South Africa, 18–21 September 2017. [Google Scholar]
- Thamaga-Chitja, J.M.; Morojele, P. The context of smallholder farming in South Africa: Towards a livelihood asset-building framework. J. Hum. Ecol. 2014, 45, 147–155. [Google Scholar] [CrossRef]
- Pienaar, L.; Traub, L.N. Understanding the Smallholder Farmer in South Africa: Towards a Sustainable Livelihoods classification. In Proceedings of the International Conference of Agricultural Economists, Milan, Italy, 8–24 August 2015; Volume 45, p. 36. [Google Scholar]
- Banga, C.B.; Abin, S.V.C. Modelling alternative herd production models for the smallholder dairy production system in South Africa. In Proceedings of the 51st Annual Congress of SASAS, Bloemfontein, South Africa, 10–12 June 2019; pp. 9–10. [Google Scholar]
- Crawshaw, R. Animal feeds, feeding practices and opportunities for feed contamination: An introduction. In Animal Feed Contamination; Elsevier: Amsterdam, The Netherlands, 2012; pp. 11–32. ISBN 978-1-84569-725-9. [Google Scholar]
- Pettersson, H. Mycotoxin Contamination of Animal Feed; Woodhead Publishing Limited: Cambridge, UK, 2012; ISBN 9781845697259. [Google Scholar]
- Tangni, E.K.; Pussemier, L.; Hove, V.F. Mycotoxin contaminating maize and grass silages for dairy cattle feeding: Current state and challenges. J. Anim. Sci. Adv. 2013, 3, 492–511. [Google Scholar]
- Mngadi, P.T.; Govinden, R.; Odhav, B. Co-occurring mycotoxins in animal feeds. Afr. J. Biotechnol. 2008, 7, 2239–2243. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Åberg, A.T.; Haggblom, P. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins 2012, 4, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.; Skhosana, Z.D.; Motlanthe, M.; Louw, W.; Rohwer, E. Long term monitoring (2014–2018) of multi-mycotoxins in South African commercial Maize and wheat with a locally developed and validated LC-MS/MS method. Toxins 2019, 11, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Changwa, R.; Abia, W.; Msagati, T.; Nyoni, H.; Ndleve, K.; Njobeh, P. Multi-mycotoxin occurrence in dairy cattle feeds from the Gauteng province of South Africa: A pilot study using UHPLC-QTOF-MS/MS. Toxins 2018, 10, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Multi-mycotoxin screening of feed and feed raw materials from Africa. World Mycotoxin J. 2018, 11, 369–383. [Google Scholar] [CrossRef]
- Shephard, G.S.; Burger, H.M.; Gambacorta, L.; Krska, R.; Powers, S.P.; Rheeder, J.P.; Solfrizzo, M.; Sulyok, M.; Visconti, A.; Warth, B.; et al. Mycological analysis and multi mycotoxins in maize from rural subsistence farmers in the former Transkei, South Africa. J. Agric. Food Chem. 2013, 61, 8232–8240. [Google Scholar] [CrossRef] [PubMed]
- Lanyasunya, T.P.; Wamae, L.W.; Musa, H.H.; Olowofeso, O.; Lokwaleput, I.K. The Risk of Mycotoxins Contamination of Dairy Feed and Milk on Smallholder Dairy Farms in Kenya. Pakistan J. Nutr. 2005, 4, 162–169. [Google Scholar] [CrossRef]
- Kang’Ethe, E.K.; M’Ibui, G.M.; Randolph, T.F.; Lang’At, A.K. Prevalence of aflatoxin M1 and B1 in milk and animal feeds from urban smallholder dairy production in Dagoretti Division, Nairobi, Kenya. East Afr. Med. J. 2007, 84. [Google Scholar] [CrossRef]
- Kang’ethe, E.K.; Lang’a, K.A. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centres in Kenya. Afr. Health Sci. 2009, 9, 218–226. [Google Scholar] [PubMed]
- Makau, C.M.; Matofari, J.W.; Muliro, P.S.; Bebe, B.O. Association of on-farm feeds handling practices with fungal growth and Mycotoxin production on feeds in smallholder dairy farms, Nakuru, Kenya. Afr. J. Agric. Res. 2016, 11, 3741–3750. [Google Scholar] [CrossRef] [Green Version]
- Kagera, I.; Kahenya, P.; Mutua, F.; Anyango, G.; Kyallo, F.; Grace, D.; Lindahl, J. Status of aflatoxin contamination in cow milk produced in smallholder dairy farms in urban and peri-urban areas of Nairobi County: A case study of Kasarani sub-county, Kenya. Infect. Ecol. Epidemiol. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Parliament and the Council of the EU. Directive EC 2002/Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Communities Decis. 2002, 221, 8–36. [Google Scholar]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Agriculture, Forestry and Fisheries (DAFF). Fertilizers, Farm Feeds, Agricultural Remedies and Stock Remedies Act, 1947 (Act No. 36 of 1947): Farm Feeds Regulations: Amendment. Gov. Gaz. 2010, 12, 4–18. [Google Scholar]
- Verstraete, F. Risk management of undesirable substances in feed following updated risk assessments. Toxicol. Appl. Pharmacol. 2013, 270, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.; Braber, S.; Akbari, P.; Kraneveld, A.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol and its modified forms: Are there major differences? Toxins 2016, 8, 334. [Google Scholar] [CrossRef] [Green Version]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef]
- Rodrigues, I.; Handl, J.; Binder, E.M. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the Middle East And Africa. Food Addit. Contam. Part B Surveill. 2011, 4, 168–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekwomadu, T.I.; Dada, T.A.; Nleya, N.; Gopane, R.; Sulyok, M.; Mwanza, M. Variation of fusarium free, masked, and emerging mycotoxin metabolites in maize from Agriculture Regions of South Africa. Toxins 2020, 12, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driehuis, F. Silage and the safety and quality of dairy foods: A review. Agric. Food Sci. 2013, 22, 16–34. [Google Scholar] [CrossRef]
- Shephard, G.S.; van der Westhuizen, L.; Sewram, V.; van Zyl, J.; Rheeder, J.P. Occurrence of the C-series fumonisins in maize from the former Transkei region of South Africa. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef]
- Dutton, M.F.; Mwanza, M.; De Kock, S.; Khilosia, L.D. Mycotoxins in South African foods: A case study on aflatoxin M1 in milk. Mycotoxin Res. 2011, 28, 17–23. [Google Scholar] [CrossRef]
- Ndlovu, C.S.; Dutton, M.F. A survey of South African silage for fungi and mycotoxins. Afr. J. Agric. Res. 2013, 8, 4299–4307. [Google Scholar] [CrossRef]
- SAGL (South African Grain Laboratories). South African Maize Crop Quality Report 2018/2019 Season; SAGL (South African Grain Laboratories): Pretoria, South Africa, 2019; Volume 1. [Google Scholar]
- Mongkon, W.; Sugita-Konishi, Y.; Chaisri, W.; Suriyasathaporn, W. Aflatoxin B1 contamination of dairy feeds after storage in farm practice in tropical environment. Biocontrol. Sci. 2017, 22, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Sanchis, V.; Magan, N. Environmental conditions affecting mycotoxins. Mycotoxins Food 2004, 174–189. [Google Scholar] [CrossRef]
- Shephard, G.S. Special issue: Human health issues. World Mycotoxin J. 2010, 3, 313–314. [Google Scholar] [CrossRef]
- Rasmussen, R.R.; Storm, I.M.L.D.; Rasmussen, P.H.; Smedsgaard, J.; Nielsen, K.F. Multi-mycotoxin analysis of maize silage by LC-MS/MS. Anal. Bioanal. Chem. 2010, 397, 765–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Zachariasova, A.; Pospichalova, M.; Florian, M.; Hajslova, J. Occurrence of multiple mycotoxins in European feedingstuffs, assessment of dietary intake by farm animals. Anim. Feed Sci. Technol. 2014, 193. [Google Scholar] [CrossRef]
- McElhinney, C.; Danaher, M.; Elliott, C.; O’Kiely, P. Mycotoxin occurrence on baled and pit silages collected in Co. Meath. Irish J. Agric. Food Res. 2015, 54, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Veršilovskis, A.; de Saeger, S. Sterigmatocystin: Occurrence in foodstuffs and analytical methods—An overview. Mol. Nutr. Food Res. 2010, 54, 136–147. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Bandyopadhyay, R.; Sulyok, M.; Warth, B.; Krska, R. Fungal and bacterial metabolites in commercial poultry feed from Nigeria. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 1288–1299. [Google Scholar] [CrossRef]
- Nichea, M.J.; Palacios, S.A.; Chiacchiera, S.M.; Sulyok, M.; Krska, R.; Chulze, S.N.; Torres, A.M.; Ramirez, M.L. Presence of multiple mycotoxins and other fungal metabolites in native grasses from a wetland ecosystem in Argentina intended for grazing cattle. Toxins 2015, 7, 3309–3329. [Google Scholar] [CrossRef] [Green Version]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria Mycotoxins in Food and Feed: An Overview. J. Food Qual. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Stoev, S.D.; Dutton, M.F.; Njobeh, P.B.; Mosonik, J.S.; Steenkamp, P.A. Mycotoxin nephropathy in Bulgarian pigs and chickens: Complex aetiology and similarity to Balkan Endemic Nephropathy. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 72–88. [Google Scholar] [CrossRef] [Green Version]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kangethe, E.K.; Faas, J.; Lindahl, J.F.; Gathumbi, J.K. A review of the impact of mycotoxins on dairy cattle health: Challenges for food safety and dairy production in sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Milićević, D.R.; Škrinjar, M.; Baltić, T. Real and perceived risks for mycotoxin contamination in foods and feeds: Challenges for food safety control. Toxins 2010, 2, 572–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monbaliu, S.; Van Poucke, C.; Detavernier, C.T.L.; Dumoultn, F.; Van Velde, M.D.E.; Schoeters, E.; Van Dyck, S.; Averkieva, O.; Van Peteghem, C.; De Saeger, S. Occurrence of mycotoxins in feed as analyzed by a multi-mycotoxin LC-MS/MS method. J. Agric. Food Chem. 2010, 58, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.T.; Danzer, K.; Townshend, A. Use of the terms “recovery” and “apparent recovery” in analytical procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 2003, 74, 2201–2205. [Google Scholar] [CrossRef]


| Compound Feeds | Forage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mycotoxin | Calibration Range (µg/kg) | Method Validated CCα (µg/kg) | Calibration Equation Y= | R2 | Apparent Recovery (Mean ± SD) (%) | LOD (µg/kg) | LOQ (µg/kg) | Calibration Equation Y= | R2 | Apparent Recovery (Mean ± SD) (%) | LOD (µg/kg) | LOQ (µg/kg) |
| NIV | 137.5–550 | 36.22 | 0.00071x + 0.02352 | 0.983 | 101.57 ± 10.30 | 70.4 | 140.8 | 0.00074x + 0.00137 | 0.978 | 101.12 ± 11.32 | 67.5 | 135.0 |
| DON | 200–800 | 60.61 | 0.004705x − 0.04196 | 0.994 | 99.99 ± 3.80 | 58.9 | 117.7 | 0.005306x − 0.1856 | 0.960 | 99.94 ± 10.01 | 116.4 | 232.7 |
| NEO | 62.5–250 | 8.60 | 0.007308x + 0.1189 | 0.994 | 100.89 ± 5.49 | 19.4 | 38.9 | 0.01092x − 0.07249 | 0.979 | 101.16 ± 11.23 | 36.9 | 73.9 |
| FUS-X | 100–400 | 16.58 | 0.002074x + 0.06591 | 0.990 | 101.18 ± 8.37 | 38.4 | 76.8 | 0.002034x + 0.1797 | 0.961 | 101.23 ± 14.24 | 66.2 | 132.3 |
| 3-ADON | 25–100 | 4.90 | 0.002594x − 0.01132 | 0.993 | 98.98 ± 6.79 | 8.2 | 16.4 | 0.00288x + 0.06729 | 0.982 | 101.66 ± 11.57 | 18.9 | 37.7 |
| 15-ADON | 12.5–50 | 3.07 | 0.002643x + 0.02861 | 0.985 | 100.02 ± 5.95 | 6.04 | 12.0 | 0.00689x − 0.01571 | 0.994 | 99.08 ± 5.97 | 2.3 | 4.7 |
| AFG2 | 12.5–50 | 2.39 | 0.03527x + 0.07185 | 0.989 | 99.47 ± 7.06 | 5.1 | 10.2 | 0.04676x − 0.02576 | 0.965 | 100.08 ± 9.97 | 6.9 | 13.7 |
| AFG1 | 10–40 | 1.93 | 0.0486x − 0.01739 | 0.997 | 99.67 ± 4.14 | 2.1 | 4.2 | 0.06081x + 0.01183 | 0.995 | 99.71 ± 3.09 | 0.9 | 1.7 |
| AFB2 | 7.5–30 | 1.53 | 0.05322x + 0.04908 | 0.989 | 99.42 ± 7.44 | 3.0 | 6.0 | 0.05568x + 0.01857 | 0.958 | 99.78 ± 9.63 | 4.2 | 8.4 |
| AFB1 | 10–40 | 6.75 | 0.04460x + 0.04191 | 0.987 | 99.21 ± 8.59 | 4.4 | 8.8 | 0.07617x − 0.36928 | 0.998 | 99.96 ± 2.26 | 4.5 | 9.0 |
| DAS | 2.5–10 | 0.67 | 0.02847x − 0.002919 | 0.956 | 101.50 ± 10.25 | 2.0 | 4.0 | 0.04995x − 0.07476 | 0.944 | 103.42 ± 18.42 | 3.1 | 6.1 |
| AOH | 50–200 | 11.98 | 0.003834x + 0.09500 | 0.998 | 100.39 ± 2.66 | 8.8 | 17.6 | 0.01428x + 0.56141 | 0.913 | 96.40 ± 16.46 | 35.5 | 71.1 |
| HT2 | 100–400 | 9.23 | 0.000166x − 0.003306 | 0.971 | 99.46 ± 10.28 | 66.9 | 133.9 | - | - | - | - | - |
| FB1 | 250–1000 | 31.84 | 0.005176x + 0.37395 | 0.974 | 98.27 ± 13.88 | 158.8 | 317.7 | 0.005004x − 0.256436 | 0.943 | 100.20 ± 12.80 | 190.3 | 380.6 |
| T2 | 100–400 | 9.38 | 0.000353x + 0.01336 | 0.978 | 98.58 ± 12.25 | 57.7 | 115.4 | - | - | - | - | - |
| FB3 | 150–600 | 23.18 | 0.008669x+ 0.66442 | 0.994 | 99.72 ± 3.40 | 44.6 | 89.2 | 0.01194x + 0.173292 | 0.988 | 100.74 ± 8.05 | 65.2 | 130.4 |
| OTA | 37.5–150 | 3.44 | 0.004142x − 0.00402 | 0.980 | 98.42 ± 12.25 | 20.9 | 41.9 | 0.00903x + 0.020303 | 0.993 | 99.84 ± 3.88 | 5.8 | 11.7 |
| FB2 | 175–700 | 24.37 | 0.00489x + 0.07337 | 0.989 | 98.78 ± 7.92 | 72.7 | 145.3 | 0.01021x − 0.34471 | 0.977 | 100.01 ± 7.63 | 71.2 | 142.6 |
| AME | 100–400 | 17.75 | 0.00051x − 0.01590 | 0.964 | 98.30 ± 10.95 | 74.9 | 149.8 | 0.002131x + 0.27616 | 0.930 | 96.83 ± 23.57 | 78.3 | 156.7 |
| STERIG | 25–100 | 4.75 | 0.01245x + 0.081945 | 0.989 | 99.83 ± 4.88 | 10.4 | 20.7 | 0.05295x + 1.5101 | 0.980 | 98.36 ± 12.08 | 14.3 | 28.6 |
| ROQ-C | 5–20 | 1.08 | 0.00227x − 0.007229 | 0.957 | 101.29 ± 15.52 | 4.1 | 8.3 | 0.02645x + 0.00973 | 0.942 | 98.47 ± 20.14 | 1.0 | 2.0 |
| ZEN | 60–240 | 17.85 | 0.000375x + 0.01078 | 0.788 | 94.15 ± 17.47 | 120.7 | 241.4 | 0.00223x + 0.17914 | 0.957 | 99.57 ± 11.82 | 70.5 | 141.0 |
| ** ENN B | 40–160 | - | 0.004967x + 0.05773 | 0.932 | 99.61 ± 12.65 | 41.9 | 83.8 | 0.05929x − 0.79199 | 0.933 | 96.78 ± 21.58 | 40.4 | 80.7 |
| Compound Feeds (N = 31) | Forages (N = 46) | Total (N = 77) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (pos) | Concentration (µg/kg) | n (pos) | Concentration (µg/kg) | n (%) pos | Concentration (µg/kg) | |||||||
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | ||||
| NIV | 0 | nd | nd | nd | 4 | <CCα | 36.9 | 36.9 | 4 (5.2) | <CCα | 36.9 | 36.9 |
| DON | 27 | <CCα | 766.2 | 412.9 | 22 | <CCα | 2385.4 | 618.3 | 49 (63.6) | <CCα | 2385.4 | 477.7 |
| 3ADON | 10 | 8.4 | 70.1 | 32.8 | 3 | <CCα | 300.0 | 169.2 | 13 (16.9) | <CCα | 300.0 | 55.5 |
| 15ADON | 14 | 16.0 | 235.5 | 122.6 | 2 | 138.6 | 858.8 | 498.7 | 16 (20.8) | 16.0 | 858.8 | 169.6 |
| ZEN | 3 | 293.9 | 1303.9 | 636.1 | 4 | 96.7 | 1793.7 | 688.5 | 7 (9.1) | 96.7 | 1793.7 | 666.0 |
| AFG2 | 0 | nd | nd | nd | 1 | 11.1 | 11.1 | 11.1 | 1 (1.3) | 11.1 | 11.1 | 11.1 |
| AFG1 | 1 | 17.2 | 17.2 | 17.2 | 1 | 23.1 | 23.1 | 23.1 | 2 (2.6) | 17.2 | 23.1 | 20.2 |
| AFB2 | 2 | 2.2 | 4.3 | 3.2 | 1 | 6.8 | 6.8 | 6.8 | 3 (3.9) | 2.2 | 6.8 | 4.4 |
| AFB1 | 2 | <CCα | 30.2 | 30.2 | 1 | 21.9 | 21.9 | 21.9 | 3 (3.9) | <CCα | 30.2 | 26.1 |
| DAS | 0 | nd | nd | nd | 1 | 3.4 | 3.4 | 3.4 | 1 (1.3) | 3.4 | 3.4 | 3.4 |
| AOH | 7 | 17.9 | 683.4 | 181.3 | 26 | 15.5 | 3088.2 | 305.6 | 33 (42.8) | 15.5 | 3088.2 | 279.2 |
| FB1 | 15 | 71.3 | 485.2 | 208.9 | 3 | <CCα | 55.4 | 47.4 | 18 (23.4) | <CCα | 485.2 | 189.8 |
| FB2 | 11 | 66.4 | 416.2 | 168.8 | 4 | 21.1 | 50.5 | 32.3 | 15 (19.5) | 21.1 | 416.9 | 132.4 |
| FB3 | 1 | <CCα | <CCα | <CCα | 0 | nd | nd | nd | 1 (1.3) | <CCα | <CCα | - |
| OTA | 3 | 19.4 | 187.9 | 85.6 | 0 | nd | nd | nd | 3 (3.9) | 19.4 | 187.9 | 85.6 |
| AME | 0 | nd | nd | nd | 5 | <CCα | 603.2 | 229.2 | 5 (6.5) | <CCα | 603.2 | 229.2 |
| STERIG | 10 | 6.8 | 46.1 | 17.4 | 25 | <CCα | 139.1 | 31.6 | 35 (45.5) | <CCα | 139.1 | 25.8 |
| ROQC | 1 | 699.9 | 699.9 | 699.9 | 1 | 54.4 | 54.4 | 54.4 | 2 (2.6) | 54.4 | 699.9 | 377.2 |
| ** ENN | 13 | <CCα | 14230.4 | 2296.5 | 12 | <CCα | 225.2 | 93.7 | 25 (32.5) | <CCα | 14230.4 | 1143.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Changwa, R.; De Boevre, M.; De Saeger, S.; Njobeh, P.B. Feed-Based Multi-Mycotoxin Occurrence in Smallholder Dairy Farming Systems of South Africa: The Case of Limpopo and Free State. Toxins 2021, 13, 166. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020166
Changwa R, De Boevre M, De Saeger S, Njobeh PB. Feed-Based Multi-Mycotoxin Occurrence in Smallholder Dairy Farming Systems of South Africa: The Case of Limpopo and Free State. Toxins. 2021; 13(2):166. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020166
Chicago/Turabian StyleChangwa, Rumbidzai, Marthe De Boevre, Sarah De Saeger, and Patrick Berka Njobeh. 2021. "Feed-Based Multi-Mycotoxin Occurrence in Smallholder Dairy Farming Systems of South Africa: The Case of Limpopo and Free State" Toxins 13, no. 2: 166. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins13020166





