Chronic Illness Associated with Mold and Mycotoxins: Is Naso-Sinus Fungal Biofilm the Culprit?
Abstract
:1. Introduction
2. Example Case Studies
2.1. Case One
2.2. Cases Two and Three
| Patient: Source | AT a | OTA a | MT a |
|---|---|---|---|
| Father: Sinus Tissue | 1.1 | NF b | NF |
| Father: Nasal Secretions | 11.2 | 13 | NF |
| Father: Urine | NF | 18.2 | NF |
| Daughter: Sinus Tissue | 1.2 | NF | NF |
| Daughter: Nasal Secretions | NF | 3.8 | 4.68 |
| Daughter: Urine | NF | 28 | 0.23 |
3. Chronic Rhinosinusitis (CRS)
4. Detection of Mycotoxins in Invasive Aspergillosis: Humans and Animals
5. Urine Mycotoxins in CRS Patients
6. Detection of Mycotoxins from Nasal Washings, Sera and Tissues
| Study | Type of patients | Fungi present sinuses | Potential mycotoxin producing fungi in sinuses | Urine mycotoxins present | Nasal washing mycotoxins present |
|---|---|---|---|---|---|
| Ponikau [27] | Normal | Yes | Yes | ND b | ND |
| Ponikau | CRS a | Yes | Yes | ND | ND |
| Braun [28] | Normal | Yes | Yes | ND | ND |
| Braun | CRS | Yes | Yes | ND | ND |
| Murr [29] | CRS | Yes | Yes | ND | ND |
| Dennis [2] | CRS | ND | ND | Yes | ND |
| Lieberman [40] | CRS | ND | ND | Yes | ND |
| Hooper [41] | Normal | ND | ND | No | No |
| Hooper | Mold exposure | ND | ND | Yes | Yes |
| Thrasher [25] | Mold exposure | Yes | Yes | Yes | Yes |
7. Indoor Microbes and Their Fragments
8. Antifungal Therapy Directed at the Sinuses
9. Role of Biofilm
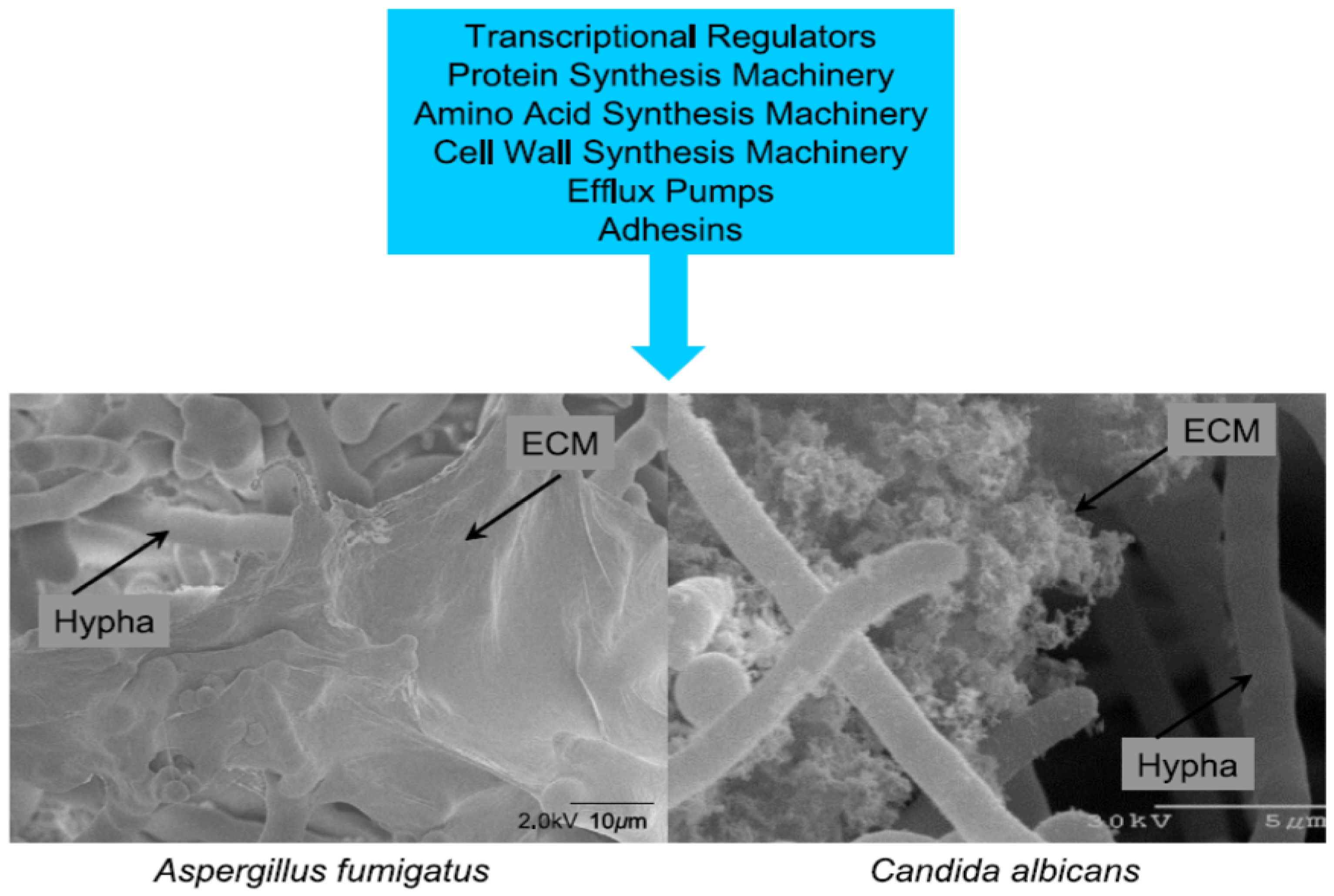
10. Conclusions
- (1)
- (2)
- (3)
- Patients that remain chronically ill (e.g., CFS) after exposure to WDB and/or mold, very commonly demonstrate mycotoxins in the urine [14,25,40,41]. Many of these patients have remained chronically ill despite leaving the moldy environment several years previous to the urine testing [14]. This suggested to us that there may well be an internal presence of toxin producing mold. We raised the question, where was the mold located in the body? Herein, we have reviewed the medical literature as it relates to the presence of fungi/mold in the nose and sinuses;
- (4)
- We reviewed data for three patients with chronic illness who required surgery for chronic fungal rhinosinusitis. Mycotoxin testing revealed the presence of AT, OTA, and MT in nasal secretions, urine and tissues samples (Table 1 and Table 2) as reported herein and by others [2,3,14,25,40,41,42]. Additionally, fungal organisms were recovered in cultures from the sinuses in these three cases including Aspergillus niger, Aspergillus fumigatus and Penicillium;
- (5)
- Humans and animals with IA have gliotoxin and aflatoxins in their sera and tissues [32,33,34,35,36,37]. These observations suggest that Aspergillus species produce mycotoxins during IA. In addition, after intratracheal administration of Stachybotrys spores, animals were found to have MT in their lungs, spleen and lymph nodes at 72 h after treatment [42]. Also, storage of mycotoxins occurs in variety of tissues [36,41,42];
- (6)
- Fungal species can be found in the sinuses of normal, healthy individuals, as well as CRS patients [27,28,55]. Species that have been recovered include those that have the capacity to produce mycotoxins. Additionally, mycotoxins (AT, OTA and MT) have been recovered from nasal washings in patients exposed to a moldy environment, however they were not found in nasal washings of healthy individuals [41];
- (7)
- The fungi that are present in the sinuses are in biofilm communities which allows for chronic persistence [39,60,61,65,66,67,68]. This would explain the chronic nature of the fungi/mold in the sinuses and explain the difficulty in treatment [39,64,83]. However, despite that, studies have demonstrated success with treating patients with intranasal amphotericin B. This was shown in both CRS patients and those with chronic illness following mold exposure [50,51,63]. Amphotericin B has been shown to have superior activity in biofilm models as opposed to other antifungal agents [50,51,84];
- (8)
- Fungal fragments from 0.03 to 0.3 microns are shed from fungal colonies known to contain antigens and toxins [44,45,46,47,48]. Fine particulates shed by Stachybotrys contain MT [18,19]. The fragments are readily deposited in the nasal cavity [46]. MT have been detected in the sera of occupants exposed to Stachybotrys [42];
- (9)
- Prior exposure to toxic mold and mycotoxins may represent an important feature of chronically ill patients such as CFS as well as those with CRS. An internal reservoir of toxin producing mold (e.g., sinuses) that persists in biofilms could produce and release mycotoxins. This model of fungal persistence may help explain these chronic illnesses and represent a potential new understanding of mechanisms of disease that can be treated and/or lessened.
Conflicts of Interest
References
- Dennis, D.P. Chronic defective T-cells responding to superantigens, treated by reduction of fungi in the nose and air. Arch. Environ. Health 2003, 58, 433–451. [Google Scholar]
- Dennis, D.P.; Roberson, D.; Curtis, L.; Black, J. Fungal exposure endocrinopathy with growth hormone deficiency; Dennis-Robertson syndrome. Toxicol. Ind. Health 2009, 25, 669–680. [Google Scholar] [CrossRef]
- Rea, W.J.; Didriksen, N.; Simon, T.R.; Pan, Y.; Fenyves, E.J.; Griffiths, G. Effects of toxic exposure to mold and mycotoxins in building-related illnesses. Arch. Environ. Health 2003, 58, 399–405. [Google Scholar]
- Campbell, A.; Thrasher, J.D.; Gray, M.R.; Vojdani, A. Mold and mycotoxins: Effects on the neurological and immune systems in humans. Adv. Appl. Microbiol. 2004, 55, 375–398. [Google Scholar] [CrossRef]
- Gray, M.R.; Thrasher, J.D.; Crago, R.; Madison, R.A.; Arnold, L.; Campbell, A.W.; Vojdani, A. Mixed mold mycotoxicosis: Immunological changes in humans following exposure to water damaged buildings. Arch. Environ. Health 2003, 58, 410–420. [Google Scholar]
- Kilburn, K.H. Neurobehavioral and pulmonary impairment in 105 adults with indoor exposure to molds compared to 100 exposed to chemicals. Toxicol. Ind. Health 2009, 35, 681–692. [Google Scholar] [CrossRef]
- Empting, L.D. Neurologic and neuropsychiatric syndrome features of mold and mycotoxin exposure. Toxicol. Ind. Health 2009, 25, 577–581. [Google Scholar] [CrossRef]
- Park, J.H.; Cox-Ganser, J.M. Mold exposure and respiratory health in damp indoor environments. Front. Biosci. 2011, E3, 757–771. [Google Scholar] [CrossRef]
- Fisk, W.J.; Eliseeva, E.A.; Mendell, M.J. Association of residential dampness and mold with respiratory tract infections and bronchitis: A meta-analysis. Environ. Health 2010, 9, 72. [Google Scholar] [CrossRef]
- Park, J.H.; Kreiss, K.; Cox-Ganser, J.M. Rhinosinusitis and mold as risk factors for asthma symptoms in occupants of a water-damaged building. Indoor Air 2012, 22, 396–404. [Google Scholar] [CrossRef]
- Tercelj, M.; Salobir, B.; Harlander, M.; Rylander, R. Fungal exposure in homes of patients with sarcoidosis—An environmental exposure study. Environ. Health 2011, 10, 8. [Google Scholar] [CrossRef]
- Laney, A.S.; Cragin, L.A.; Blevins, L.Z.; Sumner, A.D.; Cox-Ganser, J.M.; Kreiss, K.; Moffatt, S.G.; Lohff, C.J. Sarcoidosis, asthma and asthma-like symptoms among occupants of a historically water-damaged office building. Indoor Air 2009, 19, 83–90. [Google Scholar] [CrossRef]
- Chester, A.C.; Levine, P. Concurrent sick building syndrome and chronic fatigue syndrome: Epidemic neuromyasthenia revisited. Clin. Infect. Dis. 1994, 18, S43–S48. [Google Scholar] [CrossRef]
- Brewer, J.H.; Thrasher, J.D.; Straus, D.C.; Madison, R.A.; Hooper, D. Detection of mycotoxins in patients with chronic fatigue syndrome. Toxins 2013, 5, 605–617. [Google Scholar] [CrossRef]
- Polizzi, V.; Delmulle, B.; Adams, A.; Moretti, A.; Susca, A.; Picco, A.M.; Rosseel, Y.; Kindt, R.; van Bocxlaer, J.; de Kimpe, N.; et al. JEM Spotlight: Fungi, mycotoxins and microbial volatile organic compounds in mouldy interiors from water-damaged buildings. J. Environ. Monit. 2009, 11, 1849–1858. [Google Scholar] [CrossRef]
- Smoragiewicz, W.; Cossette, B.; Boutard, A.; Kryzvstyniak, K. Trichothecene mycotoxins in the dust of ventilation systems in office buildings. Int. Arch. Occup. Environ. Health 1993, 65, 113–117. [Google Scholar] [CrossRef]
- Täubel, M.; Sulyok, M.; Vishwanath, V.; Bloom, E.; Turunen, M.; Järvi, K.; Kauhanen, E.; Krska, R.; Hyvärinen, A.; Larsson, L.; et al. Co-occurrence of toxic bacterial and fungal secondary metabolites in moisture-damaged indoor environments. Indoor Air 2011, 21, 368–375. [Google Scholar] [CrossRef]
- Gottschalk, C.; Bauer, J.; Meyer, K. Detection of Satratoxin G and H in indoor air from a water-damaged building. Mycopatholgia 2008, 166, 103–107. [Google Scholar] [CrossRef]
- Brasel, T.L.; Martin, J.M.; Carriker, C.G.; Wilson, S.C.; Straus, D.C. Detection of airborne Stachybotrys chartarum macrocyclic trichothecenes in the indoor environment. Appl. Environ. Microbiol. 2005, 71, 7376–7388. [Google Scholar]
- Thrasher, J.D.; Crawley, S. The biocontaminants and complexity of damp indoor spaces: More than meets the eyes. Toxicol. Ind. Health 2009, 25, 583–615. [Google Scholar] [CrossRef]
- Straus, D.C. Molds, mycotoxins, and sick building syndrome. Toxicol. Ind. Health 2009, 25, 617–635. [Google Scholar] [CrossRef]
- Pestka, J.J.; Yike, I.; Dearborn, D.G.; Ward, M.D.; Harkema, J.R. Stachybotrys chartarum, trichothecenes mycotoxins, and damp building-related illness: New insights into a public health enigma. Toxicol. Sci. 2008, 104, 4–26. [Google Scholar] [CrossRef]
- Fukuda, K.; Strauss, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Ruprich, J. Producers and important dietary sources of ochratoxin A and citrinin. Toxins 2013, 5, 1574–1586. [Google Scholar] [CrossRef]
- Thrasher, J.D.; Gray, M.R.; Kilburn, K.H.; Dennis, D.; Yu, A. A water-damaged home and health of occupants: A case study. J. Environ. Public Health 2012. [Google Scholar] [CrossRef]
- Dennis, D.P.; Atlanta Center for E.N.T. & Facial Plastic Center, Atlanta, GA, USA. Personal Communication, 2010.
- Ponikau, J.U.; Sherris, D.A.; Kern, E.B.; Homeburger, H.A.; Frigas, E.; Gaffey, T.A.; Roberts, G.D. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin. Proc. 1999, 74, 877–884. [Google Scholar]
- Braun, H.; Buzina, W.; Freudenschuss, F.; Beham, A.; Stammberger, H. “Eosinophilic fungal rhinosinusitis”: A common disorder in Europe? Laryngoscope 2003, 113, 264–269. [Google Scholar] [CrossRef]
- Murr, A.H.; Goldberg, A.N.; Pletcher, S.D.; Dillehay, K.; Wymer, L.J.; Vesper, S.J. Some chronic rhinosinusitis patients have elevated populations of fungi in their sinuses. Laryngoscope 2012, 122, 1438–1445. [Google Scholar] [CrossRef]
- El-Morsy, S.M.; Khafagy, Y.W.; El-Naggar, M.M.; Beih, A.A. Allergic fungal rhinosinusitis: Detection of fungal DNA in sinus aspirate using polymerase chain reaction. J. Layrngol. Otol. 2010, 124, 152–160. [Google Scholar] [CrossRef]
- Guo, C.; Ghadersohi, S.; Kephart, G.M.; Laine, R.A.; Sherris, D.A.; Kita, H.; Ponikau, J.U. Improving detection of fungi in eosinophilic mucin: Seeing what we could not see before. Otolaryngol. Head Neck Surg. 2012, 147, 943–949. [Google Scholar] [CrossRef]
- Lewis, R.E.; Wiederhold, N.P.; Chi, J.; Han, X.Y.; Komanduri, K.V.; Kontoyiannis, D.P.; Prince, R.A. Detection of gliotoxin in experimental and human aspergillosis. Infect. Immun. 2005, 73, 636–637. [Google Scholar]
- Korbel, R.; Bauer, J.; Gedek, B. Pathologico-anatomic and mycotoxicologic studies of aspergillosis in birds. Tierarit Prax 1998, 21, 134–139. [Google Scholar]
- Bauer, J.; Gaareis, M.; Bott, A.; Gedek, B. Isolation of a mycotoxin (gliotoxin) from a bovine udder infected with Aspergillus fumigatus. J. Med. Vet. Mycol. 1989, 27, 45–50. [Google Scholar] [CrossRef]
- Richard, J.L.; Debey, M.C. Production of gliotoxin during pathogenic state in turkey poults by Aspergillus fumigatus. Fresneius Mycopathol. 1995, 129, 111–115. [Google Scholar]
- Matsumara, M.; Mori, T. Detection of aflatoxins in autopsied materials from a patient infected with Aspergillus flavus. Jpn. J. Med. Mycol. 1998, 39, 267–271. [Google Scholar]
- Ohtomo, T.; Murkakoshi, S.; Sugiyama, S.; Kurata, H. Detection of aflatoxin B1 in silkworm larvae attached by an Aspergillus flavus isolate from a sericultural farm. Appl. Microbiol. 1975, 39, 1034–1035. [Google Scholar]
- Bruns, S.; Seidler, M.; Albrecht, D.; Salvenmoser, S.; Remme, N.; Hertweck, C.; Brakhage, A.A.; Kniemeyer, O.; Müller, F.M. Functional genome profiling of Aspergillus fumigatus biofilm reveals enhanced production of the mycotoxin gliotoxin. Proteomics 2010, 10, 3097–3107. [Google Scholar] [CrossRef]
- Fanning, S.; Mitchell, A.P. Fungal biofilms. PLoS Pathog. 2012, 8, 1–4. [Google Scholar]
- Lieberman, S.M.; Jacobs, J.B.; Lebowitz, R.A.; Fitzgerald, M.B.; Crawford, J.; Feigenbaum, B.A. Measurement of mycotoxins in patients with chronic rhinosinusitis. Otolaryngol. Head Neck Surg. 2011, 145, 327–329. [Google Scholar] [CrossRef]
- Hooper, D.G.; Bolton, V.E.; Guilford, F.T.; Straus, D.C. Mycotoxin detection in human samples from patients exposed to environmental molds. Int. J. Mol. Sci. 2009, 10, 1465–1475. [Google Scholar] [CrossRef]
- Brasel, T.L.; Campbell, A.W.; Demers, R.E.; Ferguson, B.S.; Fink, J.; Vojdani, A.; Wilson, S.C.; Straus, D.C. Detection of trichothecene mycotoxins in sera from individuals exposed to Stachybotrys chartarum in indoor environments. Arch. Environ. Health 2004, 59, 317–323. [Google Scholar]
- Layton, R.C.; Purdy, C.W.; Jumper, C.A.; Straus, D.C. Detection of macrocyclic trichothecene mycotoxins in a caprine (goat) tracheal instillation model. Toxicol. Ind. Health 2009, 25, 693–701. [Google Scholar] [CrossRef]
- Gorny, R.L.; Reponen, T.L.; Willeke, K.; Schmechel, D.; Robine, E.; Boissier, M.; Grinshpun, S.A. Fungal fragments as indoor air biocontaminants. Appl. Environ. Microbiol. 2002, 68, 3522–3531. [Google Scholar] [CrossRef]
- Gorny, R.L. Filamentous microorganisms and their fragments in indoor air—A review. Ann. Agric. Environ. Med. 2004, 11, 185–197. [Google Scholar]
- Cho, S.-H.; Seo, S.-C.; Schmechel, D.; Grinshpun, A.G.; Reponen, T. Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos. Environ. 2005, 39, 5454–5465. [Google Scholar] [CrossRef]
- Reponen, T.; Seo, S.-C.; Grimsley, F.; Lee, T.; Crawford, C.; Grinshpun, S.A. Fungal fragments in moldy houses: A field study in homes in New Orleans and Southern Ohio. Atmos. Environ. 2007, 41, 8140–8149. [Google Scholar] [CrossRef]
- Gorny, R.L.; Lawniczek-Walczyk, A. Effect of two aerosololization methods on the release of fungal propagules from a contaminated agar surface. Ann. Agric. Environ. Med. 2012, 19, 279–284. [Google Scholar]
- Scott, J. An Evolving Architecture: Past, Present & Future and Indoor Microbiology. In Proceedings of the Indoor Air Quality Association 15th Annual Meeting and Indoor Air Expos, Las Vegas, NV, USA, 5–7 March 2012.
- Ponikau, J.U.; Sherris, D.A.; Hirohito, K.; Kern, E.B. Intranasal antifungal treatment in 51 patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2002, 110, 862–866. [Google Scholar] [CrossRef]
- Ponikau, J.U.; Sherris, D.A.; Weaver, A.; Kita, H. Treatment of chronic rhinosinusitis with intranasal amphotericin B: A randomized, placebo-controlled, double-blind pilot trial. J. Allergy Clin. Immunol. 2005, 115, 125–131. [Google Scholar] [CrossRef]
- Kern, E.B.; Sherris, D.; Stergiou, A.M.; Katz, L.; Rosenblatt, L.C.; Ponikau, J. Diagnosis and treatment of chronic rhinosinusitis: A focus on intranasal Amphotericin B. Ther. Clin. Risk Manag. 2007, 3, 319–325. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Denning, D.W.; Ferguson, B.J.; Ponikau, J.; Buzina, W.; Kita, H.; Marple, B.; Panda, N.; Vlaminck, S.; Kauffmann-Lacroix, C.; et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies. Laryngoscope 2009, 119, 1809–1818. [Google Scholar] [CrossRef]
- Siddiqui, A.; Shah, A.A.; Bashir, S.H. Craniocerebral aspergillosis of sinonasal origin in immunocompetent patients: Clinical spectrum and outcome of 25 cases. Neurosurgery 2004, 44, 602–613. [Google Scholar] [CrossRef]
- Gosepath, J.; Brieger, J.; Vlachtsis, K.; Mann, W.J. Fungal DNA is present in tissue specimens of patients with chronic rhinosinusitis. Am. J. Pathol. 2004, 18, 9–13. [Google Scholar]
- Gray, M.R.; Progressive Health Care Group, Benson, AZ, USA. Personal communication, 2012.
- Bristol-Myers Squibb. Fungizone Product Monograph; Bristol-Myers Squibb Canada: Montreal, Canada, 2009. [Google Scholar]
- Geronikaki, A.; Fesatidou, M.; Kartsey, V.; Macaey, F. Synthesis and biological evaluation of potent antifungal agents. Curr. Top. Med. Chem. 2013, 13, 2684–2733. [Google Scholar]
- Kidane, Y.H.; Lawrence, C.; Murali, T.M. Computational approaches for discovery of common immunomodulators in fungal infections: Towards broad-spectrum immunotherapeutic interventions. BMC Microbiol. 2013, 13. [Google Scholar] [CrossRef]
- Foreman, A.; Psaltis, A.J.; Tan, L.W.; Wormald, P.J. Characterization of bacterial and fungal biofilms in chronic rhinosinusitis. Am. J. Rhinol. Allergy 2009, 23, 556–561. [Google Scholar]
- Pintucci, J.P.; Corno, S.; Garotta, M. Biofilms and infections of the upper respiratory tract. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 683–690. [Google Scholar]
- Singhal, D.; Baker, L.; Wormold, P.J.; Tan, L.W. Aspergillus fumigatus biofilm on primary human sinonasal epithelial culture. Am. J. Rhinol. Allergy 2011, 25, 219–225. [Google Scholar] [CrossRef]
- Rini, J.F.; Grant, I.H. Neurological Disease after Mold Exposure, Immune Risks & Response to Biofilm-Focused Antifungal Therapy. In Proceedings of the 52nd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy Conference, San Francisco, CA, USA, 9–12 September 2013.
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521:1–528521:14. [Google Scholar]
- Fey, P.D. Modality of bacterial growth presents unique targets: How do we treat biofilm-mediated infections. Curr. Opin. Microbiol. 2010, 13, 610–615. [Google Scholar] [CrossRef]
- Foreman, A.; Wormald, P.J. Different biofilms, different disease? A clinical outcomes study. Laryngoscope 2010, 120, 1701–1706. [Google Scholar] [CrossRef]
- Loussert, C.; Schmitt, C.; Prévost, M.C.; Balloy, V.; Fadel, E.; Philippe, B.; Kauffmann-Lacroix, C.; Latjé, J.P.; Beauvais, A. The in vivo biofilm composition of Aspergillus fumigatus. Cell. Microbiol. 2010, 12, 405–410. [Google Scholar] [CrossRef]
- Beauvais, A.; Schmidt, C.; Guadagnini, S.; Roux, P.; Perret, E.; Henry, C.; Paris, S.; Mallet, A.; Prévost, M.C.; Latejé, J.C. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell. Microbiol. 2007, 9, 1588–1600. [Google Scholar] [CrossRef]
- Hung, C.; Zhou, Y.; Pinkner, J.S.; Dodson, K.W.; Crowley, J.R.; Heuser, J.; Chapman, M.R.; Hadjifrangiskou, M.; Henderson, J.P.; Hultgren, S.J. Escherichia coli biofilms have organized complex extracellular matrix structure. MBio 2013, 4. [Google Scholar] [CrossRef]
- Gibbons, J.G.; Beauvais, A.; Beau, R.; McGary, L.; Latgé, J.P.; Rokas, A. Global transcriptome changes underlying colony growth in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 68–78. [Google Scholar] [CrossRef]
- Shopova, I.; Bruns, S.; Thywissen, A.; Kniemeyer, O.; Brakhage, A.A.; Hillmann, F. Extrinsic extracellular DNA leads to biofilm formation and colocalizes with matrix polysaccharides in the human pathogenic fungus Aspergillus fumigatus. Front. Microbiol. 2013, 4, 141. [Google Scholar]
- Muller, F.M.; Seider, M.; Beauvais, A. Aspergillus fumigatus in the clinical setting. Med. Mycol. 2011, 49 (Suppl. 1), S96–S100. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, S. Biofilm formation by Aspergillus fumigatus. Med. Mycol. 2013, in press. [Google Scholar]
- Boase, D.; Jervis-Bardy, J.; Cieland, E.; Pant, H.; Tan, L.; Wormald, P.J. Bacterial-induced epithelial damage promotes fungal biofilm formation in a sheep model of sinusitis. Int. Forum Allergy Rhinol. 2013, 3, 341–348. [Google Scholar] [CrossRef]
- Boase, S.; Valentine, R.; Singhal, D.; Tan, L.W.; Wormald, P.J. A sheep model to investigate the role of fungal biofilms in sinusitis: Fungal and bacterial synergy. Int. Forum Allergy Rhinol. 2011, 1, 340–347. [Google Scholar] [CrossRef]
- Tan, N.C.; Tran, H.B.; Foreman, A.; Jardeleza, C.; Vreudge, S.; Wormold, P.J. Identifying intracellular Staphylococcus aureus in chronic rhinosinusitis: A direct comparison of techniques. Am. J. Rhinol. Allergy 2012, 26, 444–449. [Google Scholar] [CrossRef]
- Biel, M.A.; Brown, C.A.; Levinson, R.M.; Garvis, G.E.; Paisner, H.M.; Sigel, M.E.; Tedford, T.M. Evaluation of the microbiology of chronic maxillary sinusitis. Ann. Otol. Rhinol. Laryngol. 1998, 107, 942–945. [Google Scholar]
- Aral, M.; Keleş, E.; Okur, E.; Alpay, H.C.; Yilmaz, M. The pathogenicity and antibiotic resistance of coagulase-negative Staphylococci isolated from the maxillary and ethmoid sinuses. Rhinology 2004, 42, 131–136. [Google Scholar]
- Aral, M.; Keles, E.; Kaygusuz, I. The microbiology of ethmoid and maxillary sinuses in patients with chronic rhinosinusitis. Am. J. Otolaryngol. 2003, 24, 163–168. [Google Scholar] [CrossRef]
- O’Gara, J.P.; Humphreys, H. Staphylococcus epidermidis biofilms: Importance and implications. J. Med. Microbiol. 2001, 50, 582–587. [Google Scholar]
- Mack, D.; Haeder, M.; Siemssen, N.; Laufs, R. Association of biofilm production of coagulase-negative Staphylococci with expression of a specific polysaccharide intracellular adhesion. J. Infect. Dis. 1996, 174, 881–884. [Google Scholar] [CrossRef]
- Uren, B.; Psaltis, A.; Wormold, P.J. Nasal lavage with mupirocin for the treatment of surgically recalcitrant chronic rhinosinusitis. Laryngoscope 2008, 118, 1677–1680. [Google Scholar] [CrossRef]
- Ebbens, F.A.; Scadding, G.K.; Badia, L.; Hellings, P.W.; Jorissen, M.; Mullol, J.; Cardesin, A.; Bachert, C.; van Zele, T.P.; Dijkgraaf, M.G.; et al. Amphotericin B nasal lavages: Not a solution for patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2006, 118, 1149–1156. [Google Scholar] [CrossRef]
- Mowat, E.; Butcher, J.; Lang, S.; Williams, C.; Ramage, G. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J. Med. Microbiol. 2007, 56, 1205–1212. [Google Scholar] [CrossRef]
- Mowat, E.; Lang, S.; Williams, C.; McCulloch, E.; Jones, B.; Ramage, G. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J. Antimicrob. Chemother. 2008, 62, 1281–1284. [Google Scholar] [CrossRef]
- Venkatesh, M.; Rong, L.; Raad, I.; Versalovic, J. Novel synergisitic antibiofilm combinations for salvage of infected catheters. J. Med. Microbiol. 2009, 58, 936–944. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brewer, J.H.; Thrasher, J.D.; Hooper, D. Chronic Illness Associated with Mold and Mycotoxins: Is Naso-Sinus Fungal Biofilm the Culprit? Toxins 2014, 6, 66-80. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins6010066
Brewer JH, Thrasher JD, Hooper D. Chronic Illness Associated with Mold and Mycotoxins: Is Naso-Sinus Fungal Biofilm the Culprit? Toxins. 2014; 6(1):66-80. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins6010066
Chicago/Turabian StyleBrewer, Joseph H., Jack D. Thrasher, and Dennis Hooper. 2014. "Chronic Illness Associated with Mold and Mycotoxins: Is Naso-Sinus Fungal Biofilm the Culprit?" Toxins 6, no. 1: 66-80. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins6010066





